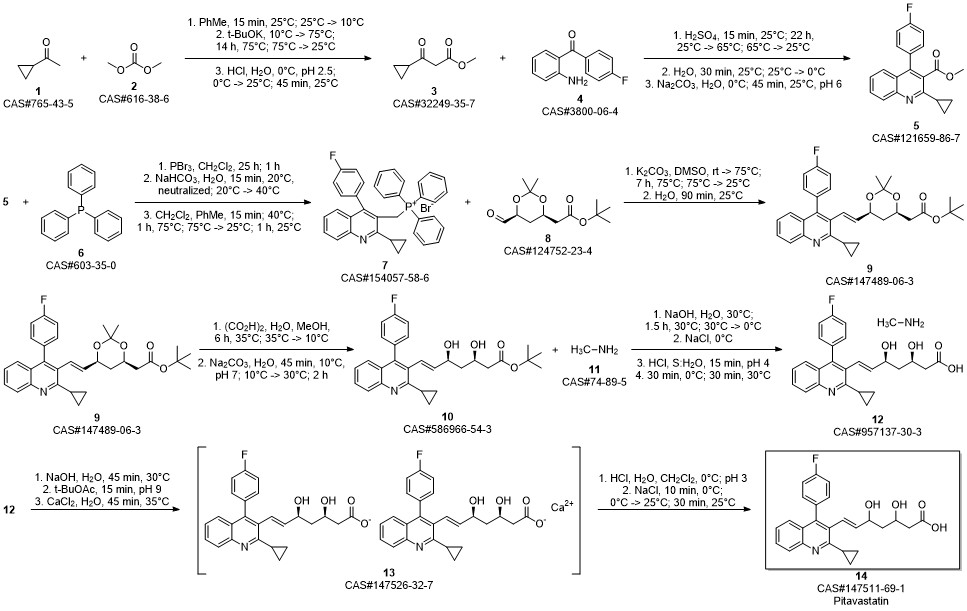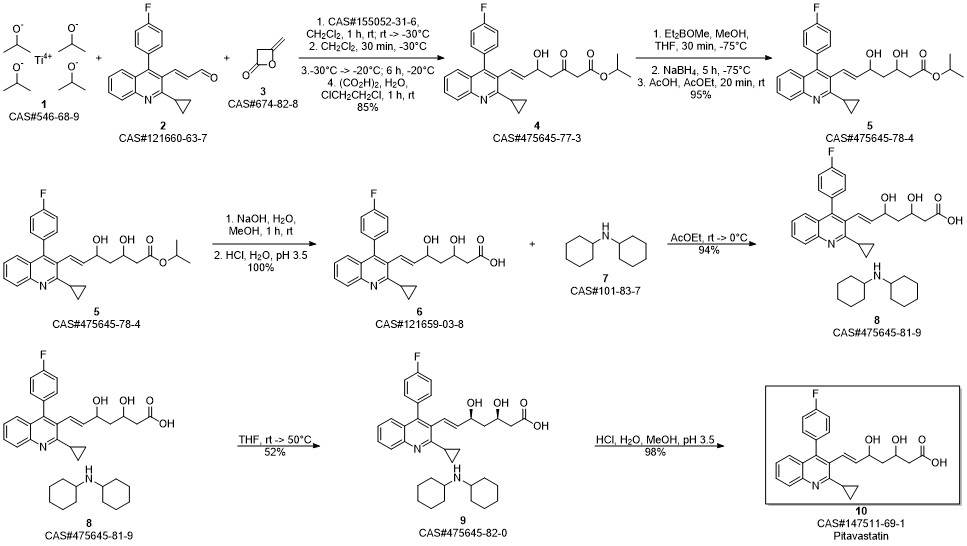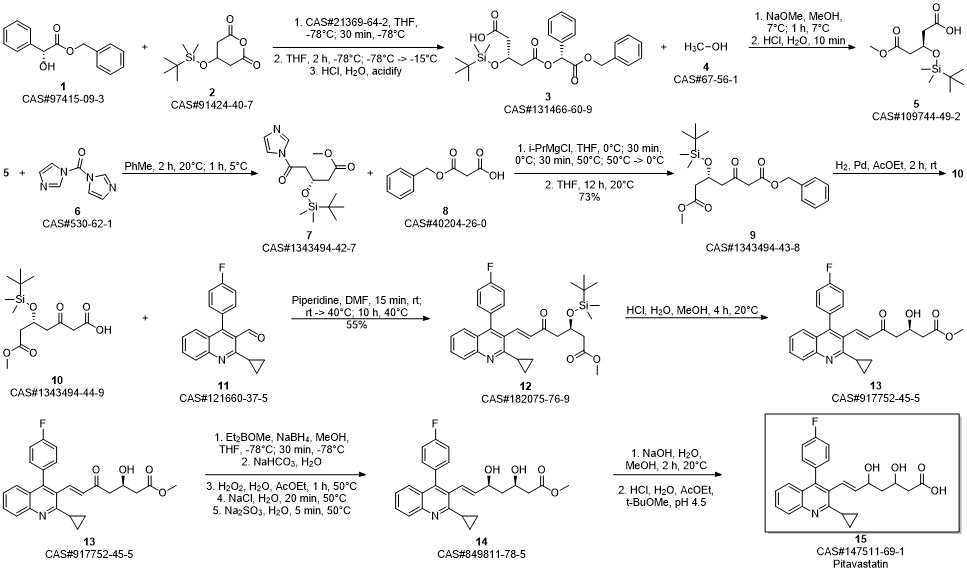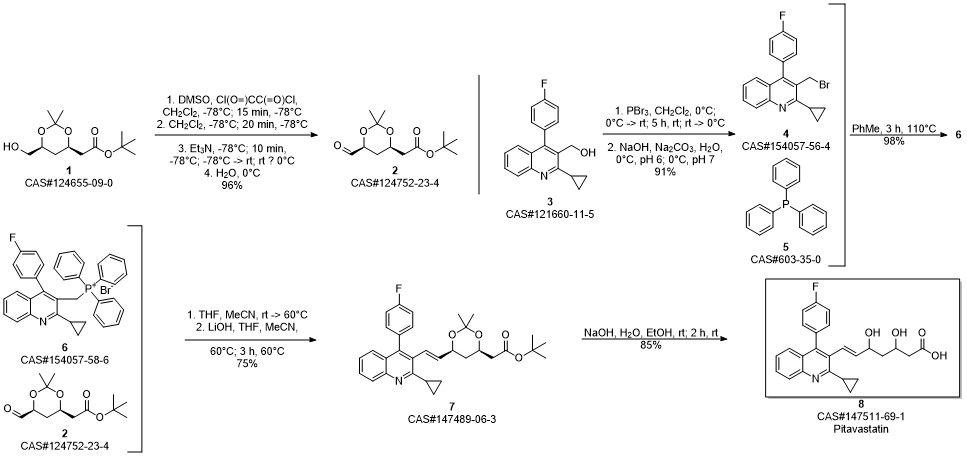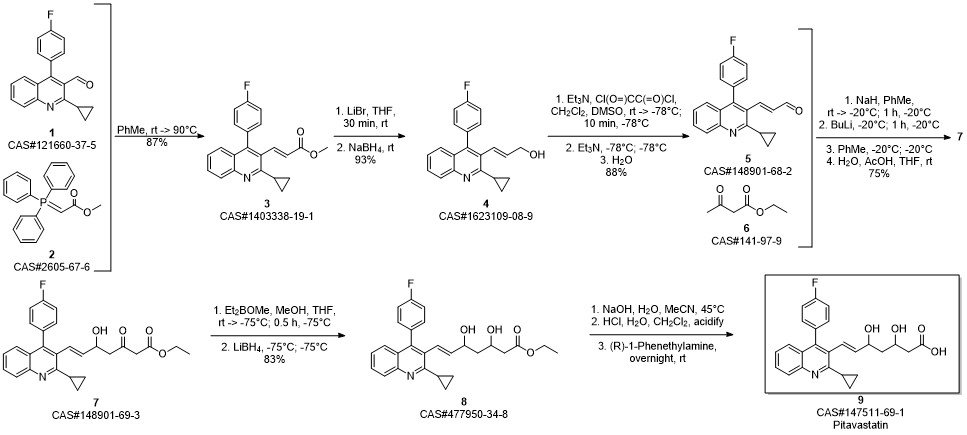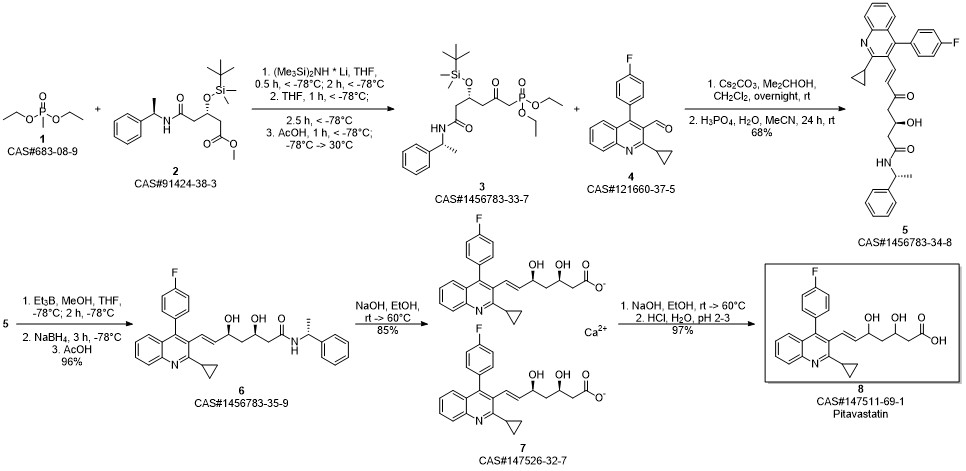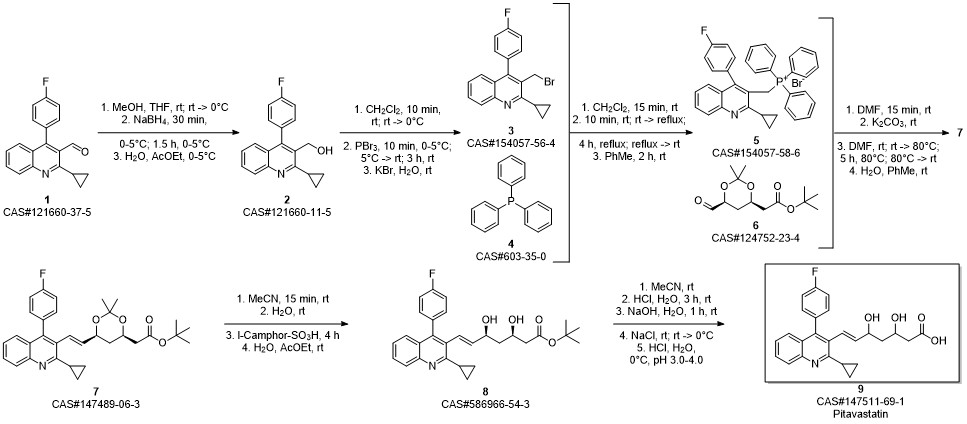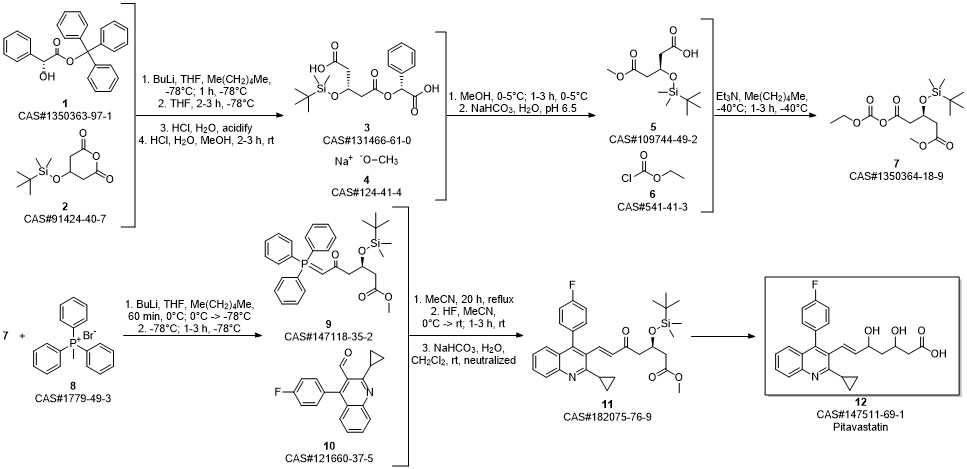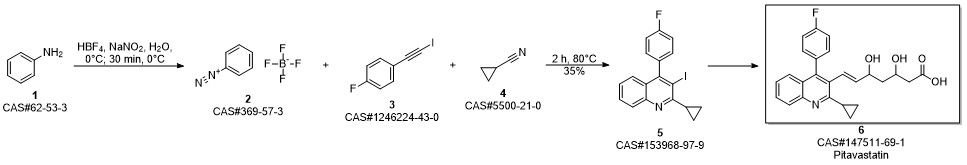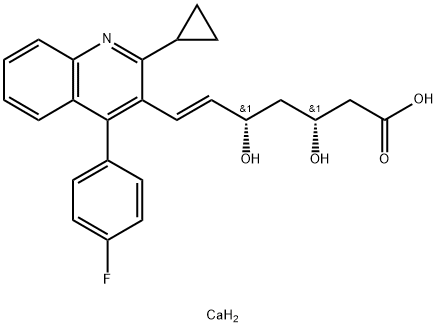
Pitavastatin calcium synthesis
- Product Name:Pitavastatin calcium
- CAS Number:147526-32-7
- Molecular formula:C25H26CaFNO4
- Molecular Weight:463.56
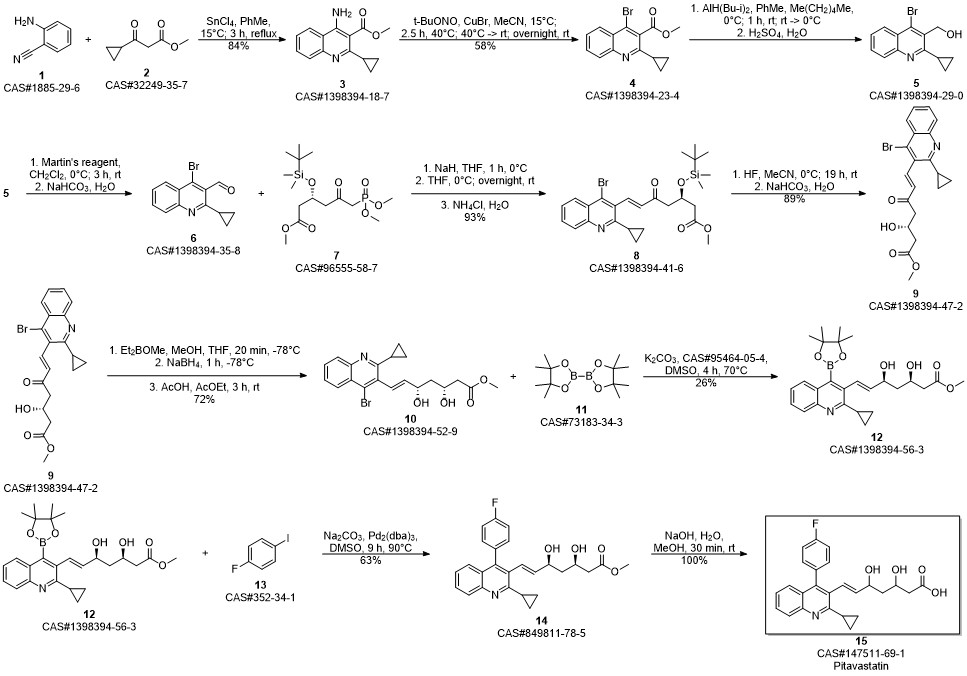
Yagi, Yusuke; Kimura, Hiroyuki; Arimitsu, Kenji; Ono, Masahiro; Maeda, Kazuya; Kusuhara, Hiroyuki; Kajimoto, Tetsuya; Sugiyama, Yuichi; Saji, Hideo. The synthesis of [18F]pitavastatin as a tracer for hOATP using the Suzuki coupling. Organic & Biomolecular Chemistry. Volume 13. Issue 4. Pages 1113-1121. 2015.
![Ethyl (E)-7-[4-(4'-fluorophenyl)-2-(cyclopropyl)-3-quinolinyl]-5-hydroxy-3-oxo-6-heptenoate](/CAS/GIF/148901-69-3.gif)
148901-69-3
113 suppliers
$98.00/100mg

147526-32-7
464 suppliers
$5.00/10mg
Yield:100 % ee
Reaction Conditions:
with Candida rugosa IFO0591 in dimethyl sulfoxide at 30; pH=7; for 20 h;Product distribution / selectivity;Aerobic conditions;potassium phosphate buffer (pH 7.0);
Steps:
16 Example 16; Production of DCOOH from 5-MOLE
Each kind of strains shown in Table 12 was incubated in the same way as that of Example 1, except that 5-MOLE was used instead of DOXE. A spot (developing solvent; hexane:ethyl acetate=1:1, Rf=0) corresponding to the compound (IV) (which is a compound, in the formula, R=hydrogen: hereinafter, abbreviated as DCOOH) on the TLC was scraped off and was then eluted with 0.25 mL of isopropanol. After centrifugation, a supernatant was subjected to a high-performance liquid chromatography (HPLC) under the same conditions as those of Example 15, to analyze the optical purity. The results are listed in Table 12
References:
US2004/30139,2004,A1 Location in patent:Page 25-26

147526-32-7
464 suppliers
$5.00/10mg

147526-32-7
464 suppliers
$5.00/10mg
![(4R,6S)-6-[(1E)-2-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]ethenyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester](/CAS/GIF/147489-06-3.gif)
147489-06-3
183 suppliers
$99.00/1g

147526-32-7
464 suppliers
$5.00/10mg
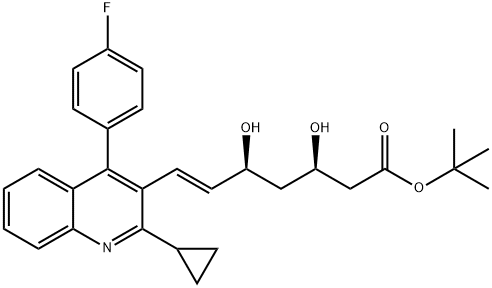
586966-54-3
152 suppliers
inquiry

147526-32-7
464 suppliers
$5.00/10mg
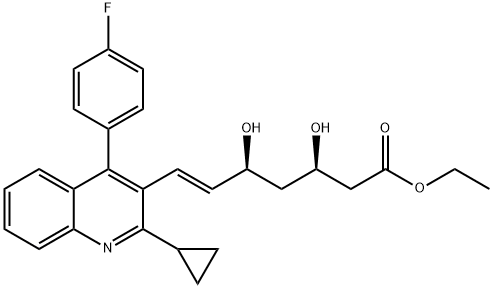
167073-19-0
54 suppliers
$742.00/250mg

147526-32-7
464 suppliers
$5.00/10mg