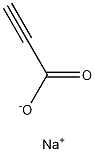
Propiolic Acid Sodium Salt synthesis
- Product Name:Propiolic Acid Sodium Salt
- CAS Number:920-38-7
- Molecular formula:C3HNaO2
- Molecular Weight:92.02861
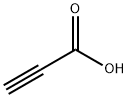
471-25-0
236 suppliers
$10.00/1g

920-38-7
25 suppliers
$130.00/500mg
Yield:920-38-7 99%
Reaction Conditions:
with sodium hydroxide in methanol at 10 - 20; for 1 h;Darkness;
Steps:
1 Preparation of Sodium Propiolate (2-Na)
Preparation of Sodium Propiolate (2-Na)
Sodium propiolate (2-Na) can be conveniently prepared in nearly quantitative yield by treating propiolic acid with sodium hydroxide in methanol (J. D. Jaufmann, et al., J. Solid State. Chem. 2000, 152, 99-104); however, sodium propiolate is somewhat light sensitive, and thus, the reaction and product isolation should be conducted using appropriate safeguards to avoid light decomposition.
In a darkened hood, ground sodium hydroxide (8.565 g, 0.214 mol) was dissolved in 1 L of methanol in a 2 L round bottom flask.
The solution was cooled to 10° C., and propiolic acid (15.0 g, 0.214 mol) was added with stirring.
The solution was stirred for 1 hour at room temperature after which the solvent was removed by rotary evaporation at a water bath temperature of ≦25° C.
A white solid product, which was dried under high vacuum, was obtained (19.50 g, 99%).
The compound was light sensitive, and thus, stored in the dark. 1H NMR (CD3OD): δ 3.36; 13C NMR (CD3OD): δ 160.8 (C=O), 81.8 (H-C≡C), 69.3 (H-C≡C).
References:
US2013/79554,2013,A1 Location in patent:Paragraph 0049