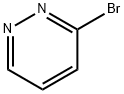
Pyridazine, 3-bromo- (9CI) synthesis
- Product Name:Pyridazine, 3-bromo- (9CI)
- CAS Number:88491-61-6
- Molecular formula:C4H3BrN2
- Molecular Weight:158.98
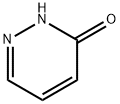
504-30-3

88491-61-6
Synthesis of 3-bromopyridazine: Bromophosphorus oxide (158 g, 552 mmol) was placed under mechanical stirring and heated to 80 °C until completely molten. Subsequently, 3-hydroxypyridazine (30.5 g, 317 mmol) was added to the molten phosphorus bromide in a single step, and the color of the reaction mixture rapidly changed from orange to yellow, and eventually formed a black solid. The reaction system was heated up to 120 °C and maintained at this temperature for 3 hours. Upon completion of the reaction, the reaction mixture was cooled to room temperature, followed by further cooling in an ice water bath. Ice water (total 300 mL) was added slowly, taking care to control the rate of addition to mitigate the exothermic phenomenon (white smoke generation was observed). During stirring, some of the solids were not dissolved, at which point 2M NaOH aqueous solution (180 mL) was added and stirring was continued for 45 minutes until all solids were completely dissolved. The resulting dark red/brown solution was slowly poured into a pre-cooled ice/water bath containing 2M NaOH aqueous solution (910 mL), keeping the internal temperature below 25°C. The solution was then mixed with 2M NaOH aqueous solution (910 mL). The pH of the solution was adjusted to about 9.5 with 2M NaOH aqueous solution (50 mL).The solution was extracted using dichloromethane (DCM, 5 x 250 mL) and the yellow organic layers were combined, dried with anhydrous sodium sulfate (Na2SO4), filtered and concentrated in vacuum to give a gray/brown solid crude product. The crude product was adsorbed on silica (98 g) and purified by silica gel column (200 g) using heptane/ethyl acetate (1:1) as eluent. Fractions containing the target product were collected, combined and concentrated in vacuum to afford 3-bromopyridazine (34.7 g, 216 mmol, 69% yield) as a green/gray solid.GCMS analysis showed product purity >99%.

504-30-3
239 suppliers
$6.00/1g

88491-61-6
140 suppliers
$11.00/250mg
Yield:88491-61-6 69%
Reaction Conditions:
with phosphorus(V) oxybromide at 80 - 120; for 3 h;
Steps:
Synthesis of 3-bromopyridazine
Synthesis of 3-bromopyridazinePhosphorous oxybromide (158 g, 552 mmol) was heated to 80 °C under mechanical stirring until molten.3-Hydroxypyridazine (30.5 g, 317 mmol) was added in one portion to the orange liquid, which affordedimmediately a yellow, then a black solid. This was heated to 120 °C and left at this temperature for 3 h. After cooling the black solid was cooled with an ice water bath, small portions of ice water (in total; 300 ml) were slowly added and an exotherm was observed (white smoke). During stirring, some solids didn’t dissolve 2 M aqueous NaOH (180 mL) was added at RT and stirred for 45 mm until all solids weredissolved. The dark red/brown solution was poured into a mechanically stirred ice/water bath, which contained 2 M aqueous NaOH solution (910 mL). The internal temperature was kept below 25 “C during the addition. The pH was adjusted to -9.5 by addition of 2 M aqueous NaOH (50 mL). The brown solution was extracted with DCM (5x 250 mL). The combined yellow organic layer was dried over Na2SO4, filtered and concentrated in vacuo to obtain a grey/brown solid. The crude material was coated on silica (98 g)and filtered with heptane/EtOAc (1:1) over a plug of silica (200 g). Product containing fractions were combined and concentrated in vacuo to afford 3-bromopyridazine (34.7 g 216 mmol, 69%) as a green/grey solid. GCMS: >99% pure.
References:
GRÜNENTHAL GMBH;NARDI, Antonio;RATCLIFFE, Paul;CRAAN, Tobias;HERTRAMPF, Thorsten;LESCH, Bernhard;KIME, Robert;STEINHAGEN, Henning WO2015/161928, 2015, A1 Location in patent:Page/Page column 68

504-30-3
239 suppliers
$6.00/1g

88491-61-6
140 suppliers
$11.00/250mg