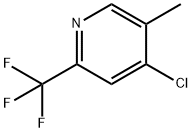
Pyridine, 4-chloro-5-methyl-2-(trifluoromethyl)- synthesis
- Product Name:Pyridine, 4-chloro-5-methyl-2-(trifluoromethyl)-
- CAS Number:220870-80-4
- Molecular formula:C7H5ClF3N
- Molecular Weight:195.57
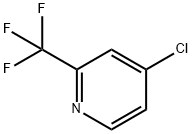
131748-14-6
175 suppliers
$15.00/1g

74-88-4
361 suppliers
$15.00/10g

220870-80-4
9 suppliers
inquiry
Yield:220870-80-4 94%
Reaction Conditions:
Stage #1: 4-chloro-2-trifluoromethylpyridinewith lithium diisopropyl amide in tetrahydrofuran at -78; for 0.5 h;
Stage #2: methyl iodide in tetrahydrofuran at -78 - 20;
Steps:
4-Chloro-5-methyl-2-(trifluoromethyl)pyridine
To a mixture of B8.2 (1.5 g, 8.3 mmol) in THF (20 mL) was added LDA (6 mL, 12 mmol) dropwise at -78 °C. The mixture was stirred at the at -78 °C for 30 min. Then methyl iodide (1.4 g, 9.96 mmol) was added. The reaction was allowed to warm to RT and stirred over night, then quenched by Ssatd. NH4Cl (15 mL), extracted with Et2O (20mL × 2). The combined organic phase was concentrated to give the title compound (1.5 g, 94%) as a yellow oil.
References:
WO2017/221092,2017,A1 Location in patent:Paragraph 00179

220870-77-9
5 suppliers
inquiry

220870-80-4
9 suppliers
inquiry

204516-19-8
0 suppliers
inquiry

220870-80-4
9 suppliers
inquiry
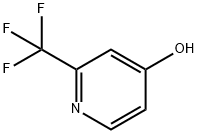
170886-13-2
172 suppliers
$16.80/250mg

220870-80-4
9 suppliers
inquiry