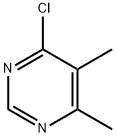
Pyrimidine, 4-chloro-5,6-dimethyl- (7CI,9CI) synthesis
- Product Name:Pyrimidine, 4-chloro-5,6-dimethyl- (7CI,9CI)
- CAS Number:67434-65-5
- Molecular formula:C6H7ClN2
- Molecular Weight:142.59
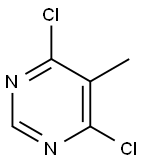
4316-97-6
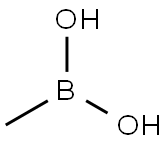
13061-96-6

67434-65-5
GENERAL STEPS: 4,6-Dichloro-5-methylpyrimidine (Sigma-Aldrich, item 595446, 0.50 g, 3.07 mmol) and tetrakis(triphenylphosphine)palladium(0) (0.18 g, 0.15 mmol) were dissolved in toluene (6 mL). Cesium carbonate (3 g, 9.20 mmol) was added to the mixture, followed by methylboronic acid (0.20 g, 3.37 mmol) and N,N-dimethylformamide (DMF, 1 mL). The reaction mixture was placed in a microwave reactor and heated by microwave radiation at 140°C (2 cycles of 30 min each). Upon completion of the reaction, it was cooled to room temperature and filtered. The solvent was removed under reduced pressure and the residue was dissolved in ethyl acetate (EtOAc) and washed sequentially with saturated aqueous sodium bicarbonate (NaHCO3) and brine. The organic phase was separated, dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated to afford the target product 4-chloro-5,6-dimethylpyrimidine (0.18 g, 1.26 mmol, 41% yield). The product was analyzed by ultra-performance liquid chromatography (UPLC) with a retention time (rt) of 0.53 min, and the major peaks observed were 143 ([M + 1-HCl], 100%) and 445 ([M + 1-HCl], 33%). The elemental analysis results were consistent with the theoretical calculated value (142) for C6H7ClN2.1H NMR (400 MHz, CDCl3) δ (ppm): 8.70 (s, 1H), 2.57 (s, 3H), 2.39 (s, 3H).

4316-97-6
199 suppliers
$8.00/1g

13061-96-6
304 suppliers
$6.00/1g

67434-65-5
45 suppliers
$83.00/100mg
Yield:67434-65-5 41%
Reaction Conditions:
with caesium carbonate;tetrakis(triphenylphosphine) palladium(0) in N,N-dimethyl-formamide;toluene at 140; for 1 h;Irradiation;
Steps:
8
To a mixture of 4,6-dichloro-5-methylpyrimidine (available from Sigma- Aldrich No.595446) (0.50 g, 3.07 mmol) and tetrakis(triphenylphosphine)palladium (0) (0.18 g, 0.15 mmol) in toluene (6 ml) and DMF (1 ml) was added cesium carbonate (3 g, 9.20 mmol), followed by methylboronic acid (0.20 g, 3.37 mmol). The resulting reaction mixture was heated at 140 0C under microwave irradiation (2 cycles x 30 min), allowed to cool down to room temperature and filtered. Solvents were removed under reduced pressure. The residue was taken-up in EtOAc and washed with a saturated NaHCO3 aqueous solution and brine. The organic phase was separated, dried (Na2SO4), filtered and concentrated to afford the title compound D8 (0.18 g, 1.26 mmol, 41% yield). UPLC: rt = 0.53 min, peak observed: 143
References:
WO2009/3997,2009,A1 Location in patent:Page/Page column 20-21
34916-78-4
53 suppliers
$45.00/50mg

67434-65-5
45 suppliers
$83.00/100mg

34916-78-4
53 suppliers
$45.00/50mg

67434-65-5
45 suppliers
$83.00/100mg
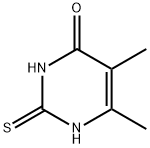
28456-54-4
72 suppliers
$48.00/500mg

67434-65-5
45 suppliers
$83.00/100mg
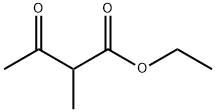
609-14-3
286 suppliers
$5.00/5g

67434-65-5
45 suppliers
$83.00/100mg