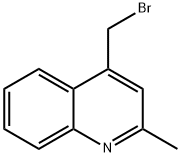
Quinoline, 4-(bromomethyl)-2-methyl- synthesis
- Product Name:Quinoline, 4-(bromomethyl)-2-methyl-
- CAS Number:864779-06-6
- Molecular formula:C11H10BrN
- Molecular Weight:236.11
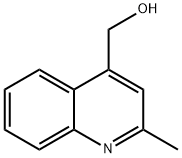
4939-28-0
47 suppliers
$53.00/250mg

864779-06-6
16 suppliers
inquiry
Yield: 94%
Reaction Conditions:
with N-Bromosuccinimide;triphenylphosphine in tetrahydrofuran at 0 - 20;
Steps:
1
4-(bromomethyl)-2-methylquinoline. To a solution of (2-methyl-quinolin-4-yl)-methanol (200 mg, 1.15 mmol) in THF (8 ml) at 0° C. was added NBS (431 mg, 2.42 mmol) and triphenylphosphine (605 mg, 2.31 mmol). The reaction mixture was allowed to warm to room temperature and stirred reaction overnight. The reaction mixture was quenched with sat NaHCO3 and extracted with ethyl acetate. The organic portions were dried over MgSO4, filtered and concentrated. Column chromatography on silica gel (10-30% ethyl acetate/hexanes) afforded intermediate 1 (258 mg, 1.09 mmol, 94%). 1H NMR (400 MHz, CDCl3) δ ppm 7.95-8.18 (m, 2H), 7.65-7.81 (m, 1H), 7.43-7.66 (m, 1H), 7.32 (s, 1H), 4.82 (s, 2H), 2.73 (s, 3H).
References:
Bristol-Myers Squibb Company US2009/18163, 2009, A1 Location in patent:Page/Page column 20