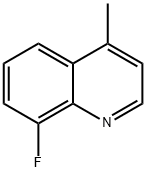
Quinoline, 8-fluoro-4-Methyl- synthesis
- Product Name:Quinoline, 8-fluoro-4-Methyl-
- CAS Number:31698-56-3
- Molecular formula:C10H8FN
- Molecular Weight:161.18
Yield:31698-56-3 65%
Reaction Conditions:
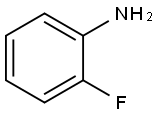
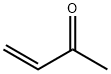
Stage #1: 2-Fluoroaniline;methyl vinyl ketonewith acetic acid at 70 - 75; for 1.33333 h;
Stage #2: zinc(II) chloride for 2 h;Heating / reflux;
Stage #3: with sodium hydroxide in water;
Steps:
8
Example 8; Preparation of 8-Fluoro-4-Methylquinoline; To a stirred solution of 2-fluoroaniline (1g. 9.0 mmol.) in acetic acid (10 ml), activated silferc (1.4g. ferric chloride 9.0 mmol) was added under nitrogen atmosphere. The reaction mixture was -stirred for 5 minutes and methyl vinyl ketone (MVK) (0.7g, 9.9 mmol) was added slowly over a period of 15 minutes. The reaction mixture was heated to 70 0C and maintained between 70-75 0C for one hour. Anhydrous zinc chloride (1.2g. 9.0 mmol) was added and the reaction was further refluxed for two hours. The reaction mixture was cooled, filtered, basified with 10% NaOH solution, extracted with ethyl acetate (3 x 20 ml), dried over Na2SO4 and evaporated to give the product; Yield 65%..
References:
WO2007/60685,2007,A1 Location in patent:Page/Page column 7-8