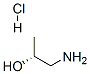
(R)-1-Amino-2-propanol hydrochloride synthesis
- Product Name:(R)-1-Amino-2-propanol hydrochloride
- CAS Number:130680-58-9
- Molecular formula:C3H10ClNO
- Molecular Weight:111.5706
72-19-5
759 suppliers
$4.76/25g

130680-58-9
12 suppliers
inquiry
Yield:130680-58-9 59%
Reaction Conditions:
Stage #1: L-threoninewith (R)-Carvone in propan-1-ol at 20 - 190; under 11251.1 Torr; for 0.0833333 h;Sealed tube;Microwave irradiation;
Stage #2: with hydrogenchloride in propan-1-ol;water at 80; for 0.0833333 h;Microwave irradiation;
Steps:
General “One-Pot” Procedure for the Decarboxylation of Amino Acids
General procedure: General “One-Pot” Procedure for the Decarboxylation of Amino Acids [0045] A magnetic stir bar, 3 mL of n-PrOH, 10 mmol of R-Carvone, and 5 mmol of amino acid were charged to a pressure vessel. The vessel was heated from room temperature to 190° C. over 5 min with stirring. If necessary the reaction vessel was maintained at 190° C. for additional time until the slurry became clear. The vessel was allowed to cool to below the solvent boiling point, carefully vented to release evolved CO2, and 10 mL of 2M HCl was added. The vessel was heated to 190° C. over 5 min with stirring and allowed to cool. The aqueous reaction mixture was washed three times with 25 mL of ether and water solvent distilled off from the hydrochloride salt. The hydrochloride salt was transferred to a vacuum oven and dried overnight at 150° C. and 10 Torr. The hydrochloride salt was then weighed and analyzed via IR and NMR.; 1-aminopropan-2-ol hydrochloride, δH 1.08 3H d J=4, 2.73 1H dd J=8, 2.95 1H dd J=12, 3.88 1H m J=4; δC 19.54, 45.46, 63.83
References:
US2014/275569,2014,A1 Location in patent:Page/Page column 0045; 0051; 0066; 0067