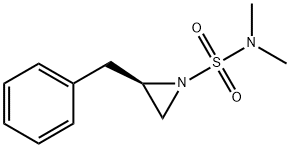
(R)-2-benzyl-N,N-diMethylaziridine-1-sulfonaMide synthesis
- Product Name:(R)-2-benzyl-N,N-diMethylaziridine-1-sulfonaMide
- CAS Number:1370406-77-1
- Molecular formula:C11H16N2O2S
- Molecular Weight:240.32
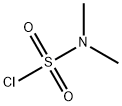
13360-57-1
293 suppliers
$30.00/5g
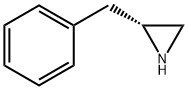
77184-95-3
28 suppliers
$110.00/250mg

1370406-77-1
11 suppliers
inquiry
Yield:1370406-77-1 76%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at -10; for 16 h;
Steps:
1
Example 1. Preparation of (i?)-2-benzyl-N,N-dimethylaziridine-l -sulfonamide (XlXa)? -2-Benzylaziridine XlXaTo a cooled (-10 °C) solution of (i?)-2-benzylaziridine (152 g, 1.14 mol) and N,N-dimethylsulfamoyl chloride (128.5 mL, 1.20 mol) in dichloromethane (762 mL) was added N.N-diisopropylethylamine (198.5 mL, 1.14 mol). The resulting yellow solution was stirred at -10 °C for a minimum of 16 hours. A 0.5M solution of citric acid (300 mL) was then added and the phases were separated. The organic phase was washed with 1.0 M sodium bicarbonate solution (300 mL). The organic phase was solvent exchanged into fert-butyl methyl ether (760 mL). The solution was cooled to 0 °C, and heptane (1.5 L) was added dropwise over a period of 2 hours. The mixture was aged for an additional 2 hours at 0 °C, then cooled to -10 °C, allowing the compound of formula XlXa to precipitate out as a white, crystalline solid (209 g, 76%). Rf: 0.53 (Si02; 1 :1 heptane:ethyl acetate, KMn04). 1H NMR (400 MHz, CDC13): δ 7.20-7.29 (m, 5H), 2.94 (dd, J= 14, 5 Hz, 1H), 2.80-2.88 (m, 1H), 2.70 (dd, J= 14, 7 Hz, 1H), 2.66 (s, 6H), 2.56 (d, J= 7 Hz, 1H), 2.14 (d, J= 4 Hz, 1H). 13C NMR (100 MHz, CDC13): δ 137.4, 129.3, 128.9, 127.2, 40.6, 38.3, 38.1, 33.0.
References:
WO2012/45007,2012,A1 Location in patent:Page/Page column 45