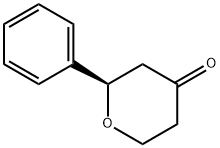
(R)-2-Phenyldihydro-2H-pyran-4(3H)-one synthesis
- Product Name:(R)-2-Phenyldihydro-2H-pyran-4(3H)-one
- CAS Number:82110-27-8
- Molecular formula:C11H12O2
- Molecular Weight:176.21
Yield:> 97 % ee , > 97 % ee
Reaction Conditions:
with Chiralcel OD-H in isopropyl alcohol at 40;Resolution of racemate;
Steps:
The racemic 2-phenyl-tetrahydro-pyran-4-one was separated into the two enantiomers by preparative chiral SFC:Method: Column: 250 x 21.2 mm Chiralcel OD-H with 5 μm particles operated at 400C. Chromatography was carried out using 25% of 0.1% (v/v) diethylamine in isopropanol as modifier, a pressure of 120 bar and a flow rate 50 ml/min. Detection at 254 nm.Upon evaporation of combined fractions containing a single enantiomer the enantiomeric excess was measured by analytical chiral SFC. Method: Column: 250 x 4.6 mm Chiralcel OD-H with 5 μm particles operated at 400C. Chromatography was carried out using 20% of 0.1% (v/v) diethylamine in isopropanol as modifier, a pressure of 200 bar and a flow rate 3 ml/min. Detection at 210 nm.This furnished: 13 : (5V2-Phenyl-tetrahydro-pyran-4-one ee > 97%, [α]D = - 73.4° (C = 1.5, CHCl3)14 : (i?)-2-Phenyl-tetrahydro-pyran-4-one ee > 97%.
References:
WO2009/124882,2009,A1 Location in patent:Page/Page column 37
147688-62-8
71 suppliers
$34.00/100mg
82110-16-5
8 suppliers
inquiry
82110-20-1
1 suppliers
inquiry

82110-27-8
2 suppliers
inquiry

82110-28-9
1 suppliers
inquiry

100-52-7
967 suppliers
$21.30/2g

82110-27-8
2 suppliers
inquiry