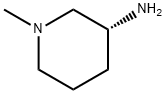
(R)-3-Amino-1-methyl-piperidine synthesis
- Product Name:(R)-3-Amino-1-methyl-piperidine
- CAS Number:1001353-92-9
- Molecular formula:C6H14N2
- Molecular Weight:114.19
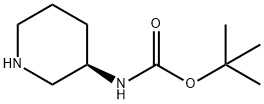
309956-78-3

1001353-92-9
The general procedure for the synthesis of 1-methyl-(R)-3-aminopiperidine from (R)-3-Boc-aminopiperidine was as follows: 1. sodium cyanoborohydride (4.51 g, 0.075 mol) was added batchwise to a mixture of tert-butyl (R)-piperidin-3-ylcarbamate (10 g, 0.05 mol), 30% aqueous formaldehyde (7.5 mL) and methanol (75 mL) at 0 °C. 2. The reaction mixture was stirred at room temperature overnight followed by vacuum concentration. 3. The residue was dissolved in a solvent mixture of ethyl acetate and water. After extraction, the organic layer was washed sequentially with water and brine and dried with anhydrous sodium sulfate. 4. Vacuum concentrating the organic layer to obtain the N-methyl compound as an oil, which can be used in the next reaction without further purification. 5. The above crude product was dissolved in methanol (60 mL) and 4N HCl in dioxane solution (10 mL) was added. 6. The reaction mixture was stirred at room temperature for 6 hours, followed by vacuum concentration. 7. The residue was ground with ether, and the resulting precipitate was filtered and washed with ice-cold methanol to yield 1-methyl-(R)-3-aminopiperidine as a solid (4.01 g, 72% yield). 1H NMR (CD3OD, 400 MHz) δ 3.54 (1H, m), 2.81 (1H, m), 2.62 (1H, m), 2.23 (3H, s), 1.97 (1H, m), 1.67-1.87 (3H, m), 1.56-1.61 (1H, m), 1.41 (9H, s), 1.15-1.42 (1H , m).

309956-78-3
551 suppliers
$11.00/5g

1001353-92-9
61 suppliers
$36.00/100mg
Yield:1001353-92-9 72%
Reaction Conditions:
with formaldehyd;sodium cyanoborohydride in methanol;water at 20;
Steps:
163.I
163I. (R)-1-methylpiperidin-3-amine Sodium cyanoborohydride (4.51 g, 0.075 mol) was added in portion to a mixture of (R)-tert-butyl piperidin-3-ylcarbamate (10 g, 0.05 mol), 30% water solution of formaldehyde (7.5 mL), and methanol (75 mL) at 0° C. The reaction mixture was stirred at room temperature overnight and concentrated in vacuo. The residue was dissolved in ethyl acetate and water. After extraction, the organic layers were washed with water, brine, and dried over Na2SO4. Concentration in vacuo, gave the N-methyl compound as an oil which was used directly without further purification. To a solution of the crude material previously obtained in methanol (60 mL) was added 4N HCl dioxane (10 mL). The reaction mixture was stirred at room temperature for 6 h. After concentration in vacuo, the residue was triturated with ether. The resulting precipitate was filtered and washed with ice-cold methanol to give the title compound as a solid (4.01 g, 72%). 1H NMR (CD3OD, 400 MHz) δ 3.54 (1H, m), 2.81 (1H, m), 2.62 (1H, m), 2.23 (3H, s), 1.97 (1H, m), 1.67-1.87 (3H, m), 1.56-1.61 (1H, m), 1.41 (9H, s), 1.15-1.42 (1H, m).
References:
Bristol-Myers Squibb Company US2008/9497, 2008, A1 Location in patent:Page/Page column 72