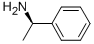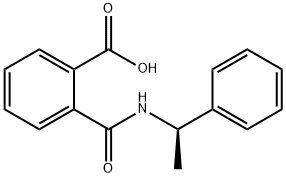
(R)-(+)-N-(1-PHENYLETHYL)PHTHALAMIC ACID synthesis
- Product Name:(R)-(+)-N-(1-PHENYLETHYL)PHTHALAMIC ACID
- CAS Number:21752-35-2
- Molecular formula:C16H15NO3
- Molecular Weight:269.3
Yield: 71%
Reaction Conditions:
in tetrahydrofuran;ethyl acetate at 5 - 10; for 15 h;
Steps:
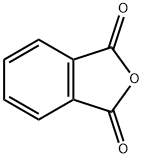
2 Synthesis of (R)-2-((1-phenylethyl)carbamoyl)benzoic acid (3).
A suspension of phthalic anhydride (50 g, 337.6 mmol) in EtOAc (200 mL) and THF (200 mL) was cooled to 5- 10° C. with stirring and carefully treated with (R)-(+)-1-phenylethylamine (47.37 mL, 371.3 mmol). After the addition was complete the reaction became clear, the ice bath was removed and the solution stirred for 15 hours. The reaction mixture was diluted with ethyl acetate (200 mL), washed with 2N HCl, saturated aqueous sodium chloride and water, dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude product was recrystallized from MTBE and hexanes to give (R)-2-((1-phenylethyl)carbamoyl)benzoic acid (3, 64.6 g, 71%) as a white powder. NMR (400 MHz,DMSO) δ 8.67 (d, J=8.2 Hz, 1H) 7.72 (dd, 1.3Hz, 1H) 7.53 (td, J=7.6, 1.4 Hz, 1H) 7.45 (td, J=7.6, 1.4 Hz, 1H) 7.38-7.35 (m, 3H) 7.27 (t, J=7.6 Hz, 2H) 7.20-7.09 (m, 1H) 5.09-5.02 (m, 1H) 1.37 (d, J=7.0 Hz, 3H).
References:
CALITHERA BIOSCIENCES, INC.;VAN ZANDT, Michael C.;SAVOY, Jennifer L. US2018/362459, 2018, A1 Location in patent:Paragraph 0171; 0172