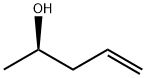
(R)-(-)-PENTEN-2-OL synthesis
- Product Name:(R)-(-)-PENTEN-2-OL
- CAS Number:64584-92-5
- Molecular formula:C5H10O
- Molecular Weight:86.13
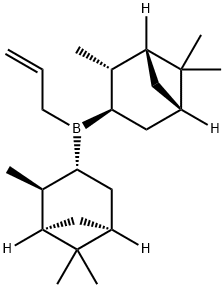
85116-38-7
13 suppliers
$76.90/5ml

75-07-0
429 suppliers
$14.00/5mL

64584-92-5
81 suppliers
$21.00/100mg
Yield: 77%
Reaction Conditions:
Stage #1:(+)-diisopinocampheylallylborane;acetaldehyde in diethyl ether at -78; for 1 h;
Stage #2: with dihydrogen peroxide;sodium hydroxide in diethyl ether;water at 25; for 2 h;
Steps:
1 4.1.1 (R)-Pent-4-en-2-ol 5
General procedure: To a stirred solution of (-)-Ipc2B(allyl)borane (150 mmol) in dry Et2O (200 mL) at -78 °C was added a solution of acetaldehyde (6 g, 136.4 mmol) in dry Et2O (20 ml). The reaction mixture was stirred at -78 °C for 1 h, after which 3 M NaOH (111 mL, 330 mmol) and 30% H2O2 (45 mL) were added to the reaction mixture and the contents were stirred at 25 °C for an additional 2 h. After completion of the reaction (monitored by TLC), the organic layer was separated and the aqueous layer extracted with Et2O, washed with brine, and dried over anhydrous Na2SO4. After removal of solvent the residue was distilled (bp 115 °C) to give (R)-(-)-4-penten-2-ol 5 (9.0 g) as a colorless liquid. Yield: 77%; [α]25D-9.67 [α]D25-9.67 (c 3.0, Et2O) {lit.7b [α]25D-9.84 [α]D25-9.84 (c 3.1, Et2O)}; IR (CHCl3): υmax 3409, 3078, 2931, 2975, 1562, 1457, 1432, 1243, 1071, 914 cm-1; 1H NMR (200 MHz, CDCl3): δ 1.19 (d, J = 6.0 Hz, 3H), 1.97 (br s, 1H), 2.14-2.26 (m, 2H), 3.83-3.84 (m, 1H), 5.10-5.14 (m, 2H), 5.76-5.87 (m, 1H); 13C NMR (50 MHz, CDCl3): δ 22.7, 43.7, 66.8, 117.9, 134.8; Anal. Calcd for C5H10O requires C, 69.72; H, 11.70. Found: C, 69.63; H, 11.78%.
References:
Shelke, Anil M.;Rawat, Varun;Suryavanshi, Gurunath;Sudalai, Arumugam [Tetrahedron Asymmetry,2012,vol. 23,# 22-23,p. 1534 - 1541]
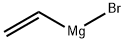
1826-67-1
209 suppliers
$51.29/100ml
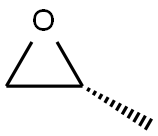
15448-47-2
197 suppliers
$50.00/1g

64584-92-5
81 suppliers
$21.00/100mg
![Silane, (1,1-dimethylethyl)dimethyl[[(1R)-1-methyl-3-buten-1-yl]oxy]-](/CAS/20210305/GIF/116773-95-6.gif)
116773-95-6
0 suppliers
inquiry

64584-92-5
81 suppliers
$21.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

64584-92-5
81 suppliers
$21.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

64584-92-5
81 suppliers
$21.00/100mg