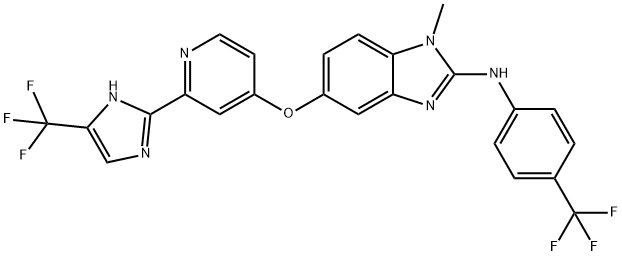
RAF265(CHIR-265) synthesis
- Product Name:RAF265(CHIR-265)
- CAS Number:927880-90-8
- Molecular formula:C24H16F6N6O
- Molecular Weight:518.41
![1,2-BENZENEDIAMINE, N1-METHYL-4-[[2-[5-(TRIFLUOROMETHYL)-1H-IMIDAZOL-2-YL]-4-PYRIDINYL]OXY]-](/CAS/GIF/927880-89-5.gif)
927880-89-5
3 suppliers
inquiry
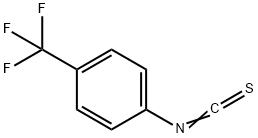
1645-65-4
141 suppliers
$10.00/1g

927880-90-8
124 suppliers
$28.00/1unit
Yield: 61%
Reaction Conditions:
Stage #1:N1-methyl-4-((2-(5-(trifluoromethyl)-1H-imidazol-2-yl)pyridin-4-yl)oxy)benzene-1,2-diamine;4-(trifluoromethyl)phenyl isothiocyanate in acetonitrile at 20; for 0.333333 h;
Stage #2: with 2-chloro-1-methyl-pyridinium iodide;triethylamine in acetonitrile at 50; for 5 h;Product distribution / selectivity;
Steps:
69 Preparation of {1-Methyl-5-[2-(5-trifluoromethyl-1H-imidazol-2-yl)-pyridin-4-yloxy]-1H-benzoimidazol-2-yl}-(4-trifluoromethyl-phenyl)-amine
EXAMPLE 69 Preparation of {1-Methyl-5-[2-(5-trifluoromethyl-1H-imidazol-2-yl)-pyridin-4-yloxy]-1H-benzoimidazol-2-yl}-(4-trifluoromethyl-phenyl)-amine 4-Trifluoromethylphenyl isothiocyanate (200 mg, 1 mmol) was added to a mixture of 4-(2-(5-(trifluoromethyl)-1H-imidazol-2-yl)pyridin-4-yloxy)-N1-methylbenzene-1,2-diamine (350 mg, 1 mmol) in 3 mL of acetonitrile. The reaction was stirred for 20 min at ambient temperature and was monitored by HPLC. Triethylamine (0.3 mL, 2.2 mmol) was added followed by 2-chloro-1-methylpyridinium iodide (270 mg, 1.05 mmol). The reaction mixture was heated to 50° C. for 5 h. The heating was stopped and 1.5 mL of water was added. After stirring the mixture for 2 h, the solid was collected by filtration and washed with 2:1 acetonitrile/water (3*1 mL) to afford 317 mg (61%) of the title compound.
References:
Novartis AG US2007/49622, 2007, A1 Location in patent:Page/Page column 41
431-35-6
218 suppliers
$10.00/5 g

927880-90-8
124 suppliers
$28.00/1unit
![2-Pyridinecarboxylic acid, 4-[4-(methylamino)-3-nitrophenoxy]-, 1,1-dimethylethyl ester](/CAS/GIF/611225-63-9.gif)
611225-63-9
4 suppliers
inquiry

927880-90-8
124 suppliers
$28.00/1unit
![2-Pyridinemethanol, 4-[4-(methylamino)-3-nitrophenoxy]-](/CAS/GIF/611226-24-5.gif)
611226-24-5
5 suppliers
inquiry

927880-90-8
124 suppliers
$28.00/1unit
![2-Pyridinecarboxaldehyde, 4-[4-(methylamino)-3-nitrophenoxy]-](/CAS/GIF/611226-25-6.gif)
611226-25-6
6 suppliers
inquiry

927880-90-8
124 suppliers
$28.00/1unit