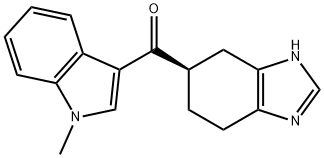
RAMOSETRON synthesis
- Product Name:RAMOSETRON
- CAS Number:132036-88-5
- Molecular formula:C17H17N3O
- Molecular Weight:279.34
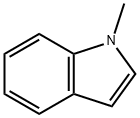
603-76-9
434 suppliers
$18.00/5g
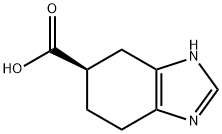
912920-60-6
0 suppliers
inquiry

132036-88-5
51 suppliers
$35.00/10mg
Yield: 95.2%
Reaction Conditions:
in tetrahydrofuran at 50; for 2 h;Microwave irradiation;Temperature;
Steps:
4 Example 4 A preparation method of ramosetron includes the following steps:
(1) Dissolve TiCl4 in a hexane solution, place the carrier particles therein and ultrasonically disperse for 5 hours.After standing for 8 hours, the hexane was distilled off, and the carrier loaded with TiCl4 was prepared after drying;(2) The TiCl4-supported carrier prepared in the previous step is dispersed in tetrahydrofuran,Add 4,5,6,7-tetrahydro-1H-benzimidazole-5-carboxylic acid mixture, heat to 50°C, microwave irradiation, and add N-methylindole dropwise under stirring conditions. Continuous response 2h,Stop the microwave irradiation after the reaction is completed, filter the temperature after it has cooled down to room temperature,Water is added to the mixture, and the mixture is shaken and mixed. Then the layers are separated by centrifugation and the organic layer is collected.And adjust the pH to 11, distill off the solvent and dry to obtain ramosetron.The carrier particles in step (1) are zeolite or vermiculite; the mass ratio of TiCl4 to carrier particles used is 1:40 g.The molar ratio of 4,5,6,7-tetrahydro-1H-benzimidazole-5-carboxylic acid to TiCl4 in step (2) is 1:2;The molar ratio of 4,5,6,7-tetrahydro-1H-benzimidazole-5-carboxylic acid to N-methyloxime is 1:1;Microwave irradiation power is 400W.The yield of ramosetron prepared was 95.2%.
References:
Suzhou Aitike Pharmaceutical Chemical Co., Ltd.;Hu Haiwei CN107880026, 2018, A Location in patent:Paragraph 0021; 0028; 0035; 0039-0045; 0049; 0056