Relebactam synthesis
- Product Name:Relebactam
- CAS Number:1174018-99-5
- Molecular formula:C12H20N4O6S
- Molecular Weight:348.38

Reference: Mangion, Ian K.; Ruck, Rebecca T.; Rivera, Nelo; Huffman, Mark A.; Shevlin, Michael. A concise synthesis of a β-lactamase inhibitor. Organic Letters. Volume 13. Issue 20. Pages 5480-5483. Journal; Online Computer File. (2011).
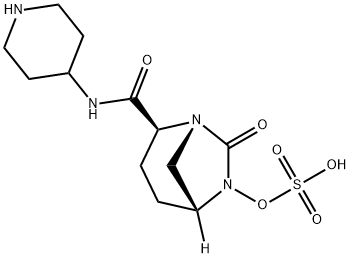
![N,N,N-tributylbutan-1-aminium [({(2S,5R)-7-oxo-2-[(piperidin-4-ylamino)carbonyl]-1,6-diazabicyclo[3.2.1]oct-6-yl}oxy)sulfonyl]oxidanide](/CAS/20210111/GIF/1174020-24-6.gif)
1174020-24-6
3 suppliers
inquiry

1174018-99-5
140 suppliers
$32.00/1mg
Yield:1174018-99-5 83.8%
Reaction Conditions:
Stage #1: tetrabutyl ammonium salt (25,5R)-4-tert-butyl{(6-sulfooxy-7-oxo-1,6-diazabicyclo[3.2.1]oct-2-ylcarbonyl)amino}piperidine-1-carboxylatewith tetrafluoroboric acid in 2,2,2-trifluoroethanol at 18 - 22; for 12.1833 h;
Stage #2: with sodium hydrogencarbonate in dichloromethane;2,2,2-trifluoroethanol;water at 18.5; pH=4.5;Product distribution / selectivity;
Steps:
1C.12
The solution of Bu4N+OSθ3 salt in TFE (34 L) was used as received from the prior step with an assumed yield of 100%. The reaction mixture was cooled in an ice bath, and HBF4-Et2θ (1.57 L) was added via addition funnel over 11 minutes between 180C and 220C.The resulting white slurry was allowed to stir overnight (12 hours). TFE (-15 L) was removed by vacuum distillation. DCM (15 L) was then added. To a 100-L extractor was charged pyrogen-free water (35 L) and NaHCθ3 (274 g), and the solution was cooled to 13°C. The reaction mixture was transferred by vacuum into the extractor with temperature of 11-130C. The reaction flask was rinsed with additional DCM (5 L) and the suspension also transferred to the extractor. The reaction mixture was warmed to 18.5 0C and de-pyrogenated water (12 L) was added to solubilize all the solids. The final pH was 4.5. The organic layer was separated, and the
References:
WO2009/91856,2009,A2 Location in patent:Page/Page column 71-72
![tert-butyl 4-((1R,2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamido)piperidine-1-carboxylate](/CAS/20180702/GIF/1174020-63-3.gif)
1174020-63-3
31 suppliers
inquiry

1174018-99-5
140 suppliers
$32.00/1mg
![1-Piperidinecarboxylic acid, 4-[[[(1R,2S,5R)-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]oct-2-yl]carbonyl]amino]-, 1-(1,1-dimethylethyl) ester](/CAS/20210305/GIF/1174020-65-5.gif)
1174020-65-5
1 suppliers
inquiry

1174018-99-5
140 suppliers
$32.00/1mg
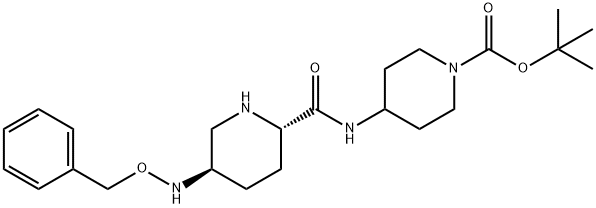
1510832-19-5
25 suppliers
inquiry

1174018-99-5
140 suppliers
$32.00/1mg
![1-?Piperidinecarboxylic acid, 4-?[[[(2S,?5R)?-?1-?[(2-?nitrophenyl)?sulfonyl]?-?5-?[[(4-?nitrophenyl)?sulfonyl]?(phenylmethoxy)?amino]?-?2-?piperidinyl]?carbonyl]?amino]?-?, 1,?1-?dimethylethyl ester](/CAS/20210305/GIF/1510832-11-7.gif)
1510832-11-7
1 suppliers
inquiry

1174018-99-5
140 suppliers
$32.00/1mg



