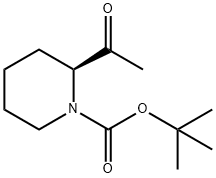
(S)-1-Boc-2-acetyl-piperidine synthesis
- Product Name:(S)-1-Boc-2-acetyl-piperidine
- CAS Number:153108-65-7
- Molecular formula:C12H21NO3
- Molecular Weight:227.3

75-16-1
275 suppliers
$12.00/10ml
![1-BOC-(2S)-[N-METHOXY-N-METHYLCARBAMOYL]PIPERIDINE](/CAS/GIF/203056-27-3.gif)
203056-27-3
17 suppliers
$110.00/1g

153108-65-7
21 suppliers
$235.00/250MG
Yield:153108-65-7 96%
Reaction Conditions:
in tetrahydrofuran at -10; for 3.5 h;Inert atmosphere;
Steps:
1.2 Synthesis of (S)-1-(tert-butoxycarbonyl)-2-acetylpiperidine 3:
Under the protection of nitrogen,dissolve 10.6 g (38.9 mmol) of compound 2 in 200 mL of tetrahydrofuran,Cool to -10°C,slowly add 32.5mL (97.3mmol) 3M methylmagnesium bromide dropwise,after 3.5 hours of reaction,add 30mL saturated aqueous ammonium chloride solution,the reaction solution was extracted with ether (60mL×3),Dry with anhydrous sodium sulfate,filter,remove the solvent under reduced pressure,Column chromatography separation,V (petroleum ether): V (ethyl acetate) = 3:1,Get 8.5g colorless oily liquid,The yield was 96%.
References:
CN111689957,2020,A Location in patent:Paragraph 0038; 0043-0046
26250-84-0
283 suppliers
$9.00/5g

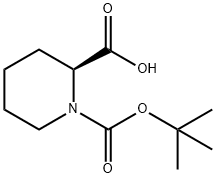
153108-65-7
21 suppliers
$235.00/250MG