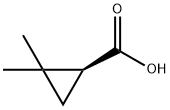
(S)-(+)-2,2-DIMETHYLCYCLOPROPANE CARBOXYLIC ACID synthesis
- Product Name:(S)-(+)-2,2-DIMETHYLCYCLOPROPANE CARBOXYLIC ACID
- CAS Number:14590-53-5
- Molecular formula:C6H10O2
- Molecular Weight:114.14

645413-67-8

14590-53-5
General procedure for the synthesis of (S)-(+)-2,2-dimethylcyclopropanecarboxylic acid from 2,2,2-difluorocyclopropanecarboxylic acid 2,2-difluoroethyl ester (60 g): in a 2L round-bottomed flask equipped with a stirrer, a pH meter, and a thermometer, 2,2,2-difluorocyclopropanecarboxylic acid 2,2-difluoroethyl ester (60 g), potassium hydrogen phosphate (2 mg) and water (600 mL) were added. Esterase (CLS-BC-21012, 1.5 g) was added to the reaction mixture and the reaction temperature was maintained at 40 °C. The pH of the reaction system was adjusted to 7.0 using 50% aqueous sodium bicarbonate.After the reaction was completed, the esterase was removed by filtration through filter paper, and 35% hydrochloric acid was slowly added to the filtrate to adjust the pH to 2.0.Subsequently, ethyl acetate (600 mL) was added to the mixture, and it was stirred for 15 min. The organic and aqueous phases were separated, and the organic phase was distilled to recover the solvent and low-boiling organics to afford (S)-(+)-2,2-dimethylcyclopropanecarboxylic acid (Yield: 49.1%, Purity: 99.0%, Optical purity: 99.1%). tlc Rf = 0.5 (n-hexane/ethyl acetate, 2:1). 1H NMR (CDCl3, 300MHz) δ: 1.55 ( dd, 1H, J = 7.95Hz, J = 5.42Hz), 1.19 (s, 3H), 1.16 (s, 3H), 1.13 (dd, 1H, J = 4.98Hz, J = 4.89Hz), 0.92 (dd, 1H, J = 7.98Hz, J = 4.42Hz).
1759-55-3
68 suppliers
$74.23/250mgs:

14590-53-5
111 suppliers
$22.00/1g
Yield: 96 % ee
Reaction Conditions:
with cell-free extract of amidase gene cloned from Klebsiella oxytoca KCTC 1686 and functionally expressed in Escherichia coli BL21(DE3) in methanolEnzymatic reaction;enantioselective reaction;
Steps:
2.9 Substrate specificity and stereoselectivity
General procedure: The substrate specificity of recombinant KamH was tested using various aliphatic and aromatic amides. Cell-free extract of E. coli BL21(DE3)/pET-Ami2 was mixed with various concentrations of substrate under standard reaction conditions; 10% (v/v) methanol was added as cosolvent to solubilize the substrate. The enzyme activity toward the various amides was determined by monitoring the formation of either carboxylic acid or ammonia [36]. Several racemic amides were used to study the stereoselectivity of the recombinant enzyme, including 2-phenylacetamide, mandelamide, 2,2-dimethylcyclopropanecarboxamide, and 2-(4-chlorophenyl)-3-methylbutyramide. Enantiomeric excess values were obtained by HPLC analysis using an AY-RH column. The enantiomeric excess and enantiomeric ratio of the product were determined according to Chen et al. [37].
References:
Guo, Fa-Mou;Wu, Jian-Ping;Yang, Li-Rong;Xu, Gang [Process Biochemistry,2015,vol. 50,# 8,p. 1264 - 1271]
1740-74-5
23 suppliers
$108.00/5g

14590-53-5
111 suppliers
$22.00/1g

1759-55-3
68 suppliers
$74.23/250mgs:

28624-52-4
8 suppliers
inquiry

14590-53-5
111 suppliers
$22.00/1g
75885-59-5
122 suppliers
$39.00/1g

14590-53-5
111 suppliers
$22.00/1g