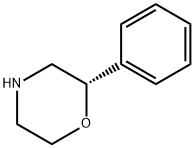
(S)-2-phenylmorpholine synthesis
- Product Name:(S)-2-phenylmorpholine
- CAS Number:74572-15-9
- Molecular formula:C10H13NO
- Molecular Weight:163.22
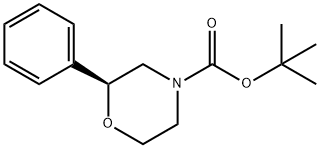
1020673-57-7

74572-15-9
General procedure for the synthesis of (S)-2-phenylmorpholine from the compound (CAS:1020673-57-7): 1.28 g of tert-butyl (S)-2-phenyl-o-phenanthroline-4-carboxylate was dissolved in 3 mL of dioxane solution in 4 N HCl and the reaction was stirred at room temperature overnight. Upon completion of the reaction, the mixture was concentrated under vacuum and subsequently diluted with 1 N HCl. The diluted aqueous phase was extracted with ether and the organic phase was discarded. The aqueous phase was adjusted to pH 12-14 with 2 N NaOH and then extracted with dichloromethane (DCM). The organic phases were combined, dried with anhydrous sodium sulfate (Na2SO4), filtered and concentrated in vacuum to give the crude product. The structure of the product was confirmed by 1H NMR analysis, resulting in 617 mg of (S)-2-phenylmorpholine in 78% yield.

1020673-57-7
2 suppliers
inquiry

74572-15-9
54 suppliers
$27.00/100mg
Yield:74572-15-9 78%
Reaction Conditions:
Stage #1: (S)-2-phenyl-morpholine-4-carboxylic acid tert-butyl esterwith hydrogenchloride in 1,4-dioxane at 20;
Stage #2: with sodium hydroxide in water; pH=12 - 14;Product distribution / selectivity;
Steps:
2
(S)-2-Phenyl-morpholine; 1.28Og (S)-2-Phenyl-niorpholine-4-carboxylic acid tert-butyl ester was dissolved in 3ImL 4N HCl in dioxane and stirred at room temperature overnight. Concentrated in vacuo and diluted with IN HCl. The aqueous was extracted with ether and then basicified to pH 12- 14 with 2N NaOH followed by extraction with DCM. The organic layers were dried over Na2SO4, filtered and concentrated in vacuo to afford 617mg of product by H NMR. 78% yield.
References:
WO2007/70760,2007,A2 Location in patent:Page/Page column 19

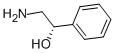
942123-04-8
1 suppliers
inquiry

74572-15-9
54 suppliers
$27.00/100mg
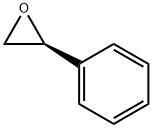
56613-81-1
146 suppliers
$22.00/250mg

74572-15-9
54 suppliers
$27.00/100mg
20780-54-5
198 suppliers
$17.00/1g

74572-15-9
54 suppliers
$27.00/100mg
![Benzenemethanol, α-[[(2-hydroxyethyl)amino]methyl]-, (αS)-](/CAS/20210305/GIF/74572-09-1.gif)
74572-09-1
0 suppliers
inquiry

74572-15-9
54 suppliers
$27.00/100mg