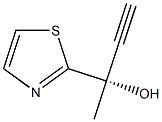
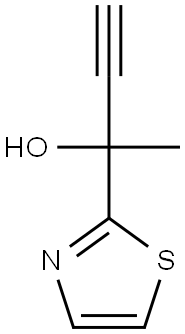
(S)-2-(thiazol-2-yl)but-3-yn-2-ol(WXC05189) synthesis
- Product Name:(S)-2-(thiazol-2-yl)but-3-yn-2-ol(WXC05189)
- CAS Number:1202769-70-7
- Molecular formula:C7H7NOS
- Molecular Weight:153.2
24295-03-2
400 suppliers
$6.00/5g
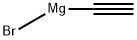
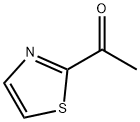
4301-14-8
126 suppliers
$68.50/100ml
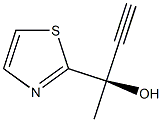
1202769-72-9
19 suppliers
inquiry

1202769-70-7
16 suppliers
inquiry
Yield:1202769-72-9 32% ,1202769-70-7 32%
Reaction Conditions:
Stage #1: 2-Acetylthiazole;acetylenemagnesium bromide in tetrahydrofuran at 20; for 2 h;Inert atmosphere;
Stage #2: with carbon dioxide;diethylamine in methanol;Supercritical conditions;Resolution of racemate;
Steps:
Intermediate 30: (R)-2-Thiazol-2-ylbut-3-yn-2-ol.
A 5 L 4-necked round-bottomed flask was charged with a solution ofethynylmagnesium bromide (1889 mL, 0.5 M in THF) under an inert atmosphere of nitrogen. To this solution was added 1-(13-thiazol-2-yl)ethan-1-one (60 g, 472 mmol) dropwise with stirring at rt. After 2 h, the resulting solution was partitioned with saturated aqueous ammonium chloride (900 mL) and water (600 mL). The resulting mixture was extracted with ethyl acetate (600 mL x 3) and the combined organic layers were washed with brine (600 mL x 2), dried over anhydrous sodium sulfate, filtered, and concentrated to dryness. The crude residue (65 g, 90%) of (R) and (S) enantiomers was further purified by chiral preparative SFC (CHIRALPAK IG 4.6 x 50 mm,3 um; mobile phase, CO2 (80%), MeOH (0.1%DEA); Detector, l = 254 nm) to afford (R)-2-(1,3-thiazol-2- yl)but-3-yn-2-ol (21 g, 32%, >97% ee) as a yellow solid and (S)-2-(1,3-thiazol-2-yl)but-3- yn-2-ol (Intermediate 31, 21 g, 32%) as a yellow solid. Data for (R)-2-(1,3-thiazol-2- yl)but-3-yn-2-ol : MS (ESI): mass calcd. for C7H7NOS, 153.0; m/z found, 153.9 [M+H]+.1H NMR (400 MHz, CDCl3) d 7.78 (d, J = 3.3 Hz, 1H), 7.36 (d, J = 3.3 Hz, 1H), 3.66 (s, 1H), 2.72 (s, 1H), 1.98 (s, 3H). [a]20D = -34.6.5 (c = 0.54 in MeOH). Intermediate 31: (S)-2-Thiazol-2-ylbut-3-yn-2-ol.The chiral separation described in Intermediate 30 provided (S)-2-(1,3-thiazol-2- yl)but-3-yn-2-ol (21 g, 32%, >97% ee) as a yellow solid. MS (ESI): mass calcd. for C7H7NOS, 153.0; m/z found, 153.9 [M+H]+.1H NMR (400 MHz, CDCl3) d 7.78 (d, J = 3.3 Hz, 1H), 7.36 (d, J = 3.3 Hz, 1H), 3.66 (s, 1H), 2.72 (s, 1H), 1.98 (s, 3H). [a]20D = +35.3 (c = 0.51 in MeOH).
References:
WO2020/239999,2020,A1 Location in patent:Page/Page column 157; 158
1202769-68-3
28 suppliers
$155.00/50mg

1202769-72-9
19 suppliers
inquiry

1202769-70-7
16 suppliers
inquiry

24295-03-2
400 suppliers
$6.00/5g

1202769-72-9
19 suppliers
inquiry

1202769-70-7
16 suppliers
inquiry