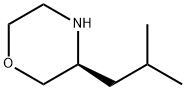
(S)-3-Isobutylmorpholine synthesis
- Product Name:(S)-3-Isobutylmorpholine
- CAS Number:77897-22-4
- Molecular formula:C8H17NO
- Molecular Weight:143.23
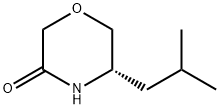
101250-44-6
6 suppliers
inquiry

77897-22-4
31 suppliers
$45.00/2.5mg
Yield:77897-22-4 95%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0; for 20.3333 h;Heating / reflux;
Steps:
67.A
Step A: To an ice cold, stirred suspension of NaH (60% in oil, 1.6 g, 39.0 mmol) in toluene (53 mL) was added dropwise a solution of (S)-2-amino-4-methylpentan-1-ol (2.0 g, 17.0 mmol) in toluene (37 mL). After the addition was completed, the reaction mixture was warmed to room temperature and a solution of ethyl chloroacetate (2.3 g, 19.0 mmol) in toluene (10 mL) was added in a dropwise manner. The resulting mixture was then stirred at reflux for 20 h, cooled to room temperature, and solid ammonium chloride (2.1 g, 39.0 mmol) was added to the reaction. The mixture was stirred for 20 min and then concentrated under reduced pressure. The crude material was purified by column chromatography (silica gel, 98:2 CH2Cl2/CH3OH to 95:5 CH2Cl2/CH3OH) to give (S)-5-iso-butylmorpholin-3-one (1.9 g, 70%) as a light yellow oil. To ice-cold THF (8 mL) was added lithium aluminum hydride (1.0 M solution in THF, 23.0 mL, 23.0 mmol). Once the addition was complete, a solution of (S)-5-iso-butylmorpholin-3-one (1.8 g, 12.0 mmol) in THF (7.0 mL) was added dropwise over 20 min. Once the addition was completed, the ice bath was removed and the reaction mixture stirred at reflux for 20 h. The reaction was cooled in an ice-bath and to this was slowly added H2O (1.2 mL), then 15% aqueous solution of sodium hydroxide (1.2 mL), and then H2O (1.2 mL). The resulting mixture was stirred at room temperature for 1.5 h and then filtered washing the solids with EtOAc (50 mL). The filtrate was concentrated at room temperature under reduced pressure to provide (S)-3-iso-butylmorpholine (1.6 g, 95%) as a light yellow oil. 1H NMR and MS consistent.
References:
US2008/255114,2008,A1 Location in patent:Page/Page column 22