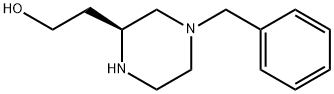
(S)-4-(Phenylmethyl)-2-piperazineethanol synthesis
- Product Name:(S)-4-(Phenylmethyl)-2-piperazineethanol
- CAS Number:477220-33-0
- Molecular formula:C13H20N2O
- Molecular Weight:220.31
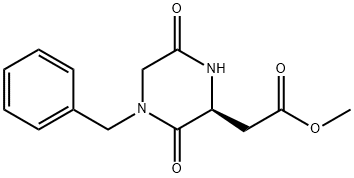
612506-99-7

477220-33-0
General procedure for the synthesis of 2-[(S)-4-benzyl-2-yl]ethanol from the compound (CAS:612506-99-7): to a tetrahydrofuran (1L) solution of methyl S-(4-benzyl-3,6-dioxopiperazin-2-yl)acetate (31.9 g, 0.12 mol) at 0 °C, lithium aluminum hydride (350 mL, 1.0 M tetrahydrofuran solution). The reaction mixture was stirred overnight at room temperature. Subsequently, the reaction was quenched by successive careful addition of 13.3 mL of water, 13.3 mL of 15% aqueous sodium hydroxide solution and 39.9 mL of water, during which vigorous stirring was maintained to ensure the formation of a fine precipitate. The reaction mixture was filtered through a sintered funnel and the solid fraction was washed well with tetrahydrofuran and dichloromethane. The filtrate was concentrated under vacuum to give 26.5 g of an oily residue which was directly sampled on a silica gel column. Column chromatographic separation was carried out using a 5% 7N ammonia-methanol dichloromethane mixture as eluent to obtain the target product, which was an orange-colored oil and solidified to a solid after vacuum drying. This solid was dissolved in acetonitrile, sonicated for several minutes and filtered to give 7.5 g (25% yield) of S-(-)-2-(4-benzylpiperazin-2-yl)ethanol as an off-white crystalline solid with a melting point of 78.9-80 °C. The mother liquor was concentrated to give 6.8 g (23% yield) of a slightly less pure product as an amorphous solid.

252873-54-4
0 suppliers
inquiry

477220-33-0
36 suppliers
inquiry
Yield: 64%
Reaction Conditions:
with lithium aluminium tetrahydride;palladium(II) hydroxide in tetrahydrofuran at -5 - 65; for 18 h;
Steps:
2-[(2S)-4-benzylpiperazin-2-yl]ethanol
Lithium aluminum hydride (13 g, 342.5 mmol) is added to a reaction vessel along with THF (200 mL). The mixture is cooled to -5-0 °C. benzyl 2-[(2S)-4-benzyl-3,6-dioxopiperazin-2-yl]acetate (50 g, 141.9 mmol) is added to a second vessel along with THF (400 mL). The solution of benzyl 2-[(2S)-4-benzyl-3,6-dioxopiperazin-2-yl]acetate is added slowly to the lithium aluminum hydride solution, keeping the temperature between -5 and 0 °C. The reaction mixture is heated to 60-65 °C and stirred for 2 hours. The mixture is cooled to 20-30 °C and stirred for 16 hours. Water (13 g) is added, then 4% NaOH in water (52 g) is added. The mixture is stirred for 1 hour, filtered, and concentrated in vacuo. Isopropyl acetate (200 mL) and 2M HC1 (150 g) are added. The layers are separated and the aqueous layer is washed again with isopropyl acetate (100 mL). The aqueous layer is adjusted to pH 11-12 with NaOH, and DCM (150 mL) is added. The layers are separated and the organic layer is washed with water (100 mL). The layers are separated and the organic layer is concentrated in vacuo to give the title compound as a solid (20.1 g, 64%). 1HNMR (CDCl3) d 7.44 - 7.20 (m, 5H), 3.81 (t, 7 = 5.2 Hz, 2H), 3.50 (s, 2H), 3.08-2.93 (m, 2H), 2.93-2.82 (m, 1H), 2.76 (d, J= 11.5 Hz, 2H), 2.02 (td, J= 11.1, 3.3 Hz, 1H), 1.85 (t, J= 10.4 Hz, 1H), 1.67 -1.52 (m, 2H).
References:
ELI LILLY AND COMPANY WO2021/118877, 2021, A1 Location in patent:Page/Page column 71-72

612506-99-7
0 suppliers
inquiry

477220-33-0
36 suppliers
inquiry
6436-90-4
374 suppliers
$8.00/10g

477220-33-0
36 suppliers
inquiry
![Glycine, N-[(1,1-dimethylethoxy)carbonyl]-L-α-aspartyl-N-(phenylmethyl)-, 2-ethyl 1-(phenylmethyl) ester (9CI)](/CAS/20211123/GIF/321918-75-6.gif)
321918-75-6
1 suppliers
inquiry

477220-33-0
36 suppliers
inquiry