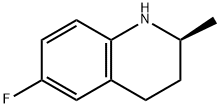
(S)-6-fluoro-2-methyl-1,2,3,4- tetrahydroquioline synthesis
- Product Name:(S)-6-fluoro-2-methyl-1,2,3,4- tetrahydroquioline
- CAS Number:199186-68-0
- Molecular formula:C10H12FN
- Molecular Weight:165.21
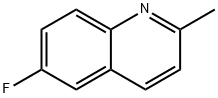
1128-61-6
203 suppliers
$8.00/250mg
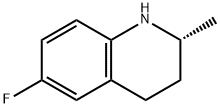
199186-69-1
9 suppliers
inquiry

199186-68-0
11 suppliers
inquiry
Yield:199186-69-1 89%
Reaction Conditions:
with formic acid;C23H35ClIrN4(1+)*Cl(1-) in water at 20; for 6 h;enantioselective reaction;
Steps:
General procedure for the asymmetric transfer hydrogenation
General procedure: A 10 mL tube was charged with quinoline (1.0 mmol), 1 mL of H2O,100 μL of the Ir catalyst solution generated in situ, and a stir bar. Themixture was stirred at RT for 6 h (or 80 .C for 2 h) and then neutralizedwith a saturated sodium bicarbonate solution. The aqueous layer wasextracted with DCM. The crude product was purified by silica gelchromatography (PE/EA) to give the desired product.
References:
Qi, Huimin;Wang, Lixian;Sun, Qiangsheng;Sun, Wei [Molecular catalysis,2022]

1128-61-6
203 suppliers
$8.00/250mg

199186-68-0
11 suppliers
inquiry
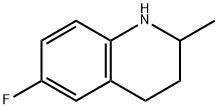
42835-89-2
90 suppliers
$20.00/100mg

199186-68-0
11 suppliers
inquiry