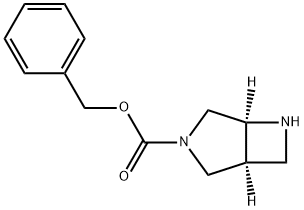
(S,S)-3-CBZ-3,6-DIAZABICYCLO[3.2.0]HEPTANE synthesis
- Product Name:(S,S)-3-CBZ-3,6-DIAZABICYCLO[3.2.0]HEPTANE
- CAS Number:370881-43-9
- Molecular formula:C13H16N2O2
- Molecular Weight:232.28

246510-70-3
2 suppliers
inquiry
![(S,S)-3-CBZ-3,6-DIAZABICYCLO[3.2.0]HEPTANE](/CAS/GIF/370881-43-9.gif)
370881-43-9
26 suppliers
$285.00/100mg
Yield:370881-43-9 97%
Reaction Conditions:
with trifluoroacetic acid in ethanol;dichloromethane;
Steps:
52.D benzyl (1S,5S)-3,6-diazabicyclo[3.2.0]heptane-3-carboxylate
EXAMPLE 52D benzyl (1S,5S)-3,6-diazabicyclo[3.2.0]heptane-3-carboxylate The product of Example 52C (43.7 g, 125 mmol) in CH2Cl2 (150 mL) was treated with trifluoroacetic acid (50 mL) at room temperature and allowed to stir for 1 hour. The mixture was concentrated under reduced pressure and the residue was dissolved in ethanol (250 mL) and basified to pH 10 with 10% aqueous NaOH. The mixture was warmed to 60° C. at 10 hours. The reaction was allowed to cool to room temperature and was concentrated under reduced pressure to remove most of the ethanol. The residue was extracted with CHCl3 (2*500 mL). The extracts were combined, washed with brine (3*50 mL) and then passed through a short column of diatomaceous earth. The filtrate was concentrated to provide the title compound as a yellow oil (28.0 g, 97%) which was used in the next step without further purification. 1H NMR (MeOH-d4, 300 MHz) δ 3.30-3.16 (m, 3H), 3.36 (m, 1H), 3.82 (m, 3H), 4.55 (m, 1H), 5.20 (s, 2H), 7.36 (m, 5H); MS (DCI/NH3) m/z 250 (M+NH4)+, 233 (M+H)+.
References:
US2002/19388,2002,A1
![1-Pyrrolidinecarboxylic acid, 3-amino-4-[[(methylsulfonyl)oxy]methyl]-, phenylmethyl ester, (3S,4S)-, mono(trifluoroacetate) (9CI)](/CAS/20211123/GIF/799279-83-7.gif)
799279-83-7
1 suppliers
inquiry
![(S,S)-3-CBZ-3,6-DIAZABICYCLO[3.2.0]HEPTANE](/CAS/GIF/370881-43-9.gif)
370881-43-9
26 suppliers
$285.00/100mg
![3-benzyl 6-tert-butyl 3,6-diazabicyclo[3.2.0]heptane-3,6-dicarboxylate](/CAS/20180629/GIF/799279-84-8.gif)
799279-84-8
2 suppliers
inquiry
![(S,S)-3-CBZ-3,6-DIAZABICYCLO[3.2.0]HEPTANE](/CAS/GIF/370881-43-9.gif)
370881-43-9
26 suppliers
$285.00/100mg
246510-69-0
10 suppliers
inquiry
![(S,S)-3-CBZ-3,6-DIAZABICYCLO[3.2.0]HEPTANE](/CAS/GIF/370881-43-9.gif)
370881-43-9
26 suppliers
$285.00/100mg

370880-75-4
34 suppliers
$206.00/1g
![(S,S)-3-CBZ-3,6-DIAZABICYCLO[3.2.0]HEPTANE](/CAS/GIF/370881-43-9.gif)
370881-43-9
26 suppliers
$285.00/100mg