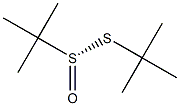
(S)-tert-Butanethiosulfinate synthesis
- Product Name:(S)-tert-Butanethiosulfinate
- CAS Number:60011-16-7
- Molecular formula:C8H18OS2
- Molecular Weight:194.36
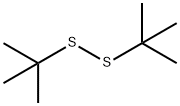
110-06-5

60011-16-7
The general procedure for the synthesis of (S)-tert-butyl sulfinic acid thio-tert-butyl ester from tert-butyl disulfide was as follows: first, ligand L2 was prepared by reacting (1R,2S)-prolinyltrimethylsilyl ether with an equimolar amount of 3,5-dimethylcarboxaldehyde (for details of the preparation method, see: Org. Synth. 2005, 82, 157). Subsequently, ligand L2 (5.0 mmol) was mixed with VO(acac)2 (1.27 g, 4.8 mmol) in acetone and the reaction was stirred for 1 hour until the system became darker in color and TLC showed that the reaction was complete. Next, diethyl tert-butyl ether (169.4 g, 0.95 mol) was dissolved in 190 mL of acetone and the temperature of the reaction system was controlled at -5°C to 0°C. At this temperature, 30% hydrogen peroxide (1.02 mol) was added slowly dropwise, and the process of dropwise addition took about 20 to 22 hours, during which time the temperature of the feedstock and the product were maintained. The reaction is considered to be at its end when the ratio of feedstock to product reaches 90:1, and the temperature should not exceed 35°C throughout the process.After completion of the reaction, the solvent is removed by distillation under reduced pressure. To the residue, 400 mL of diethoxymethane (DEM) was added for stratification. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the amount of (S)-tert-butyl thiobutyl tert-butyl tert-butyl acetyl tert-butyl sulfite in solution was determined. The product yield was 92% (169.8 g, 0.87 mol) with an enantiomeric excess (ee) value of 96.5% using acetyl tert-butyl thioester as external standard.

110-06-5
180 suppliers
$11.00/25g

60011-16-7
72 suppliers
$41.00/250mg
Yield: 92%
Reaction Conditions:
with bis(acetylacetonate)oxovanadium;C27H39NO2Si;dihydrogen peroxide in acetone at -5 - 0;
Steps:
2.2 Example 2
After reacting (1R,2S)-prolinol trisilyl ether with 3,5-dimethylsalicaldehyde in an equivalent amount, ligand L2 was obtained (specific process reference: Org. Synth. 2005, 82, 157). The ligand L2 (5.0 mmol) and VO(acac)2 (1.27 g, 4.8 mmol) were added to acetone and stirred for 1 hour. After the color of the system became dark, the TLC reaction was complete. T-butyl disulfideEthyl ether (169.4 g, 0.95 mol) was dissolved in 190 ml of acetone, and the temperature was controlled at -5°C to 0°C. 30% hydrogen peroxide (1.02 mol) was slowly added dropwise. The addition was completed in about 20-22 hours, and the raw materials and products were kept warm. When the ratio reaches 90:1, it is considered as the end of the reaction. The temperature is controlled to not exceed 35°C. The solvent is distilled off under reduced pressure. 400 mL of diethoxymethane (DEM) is added for layering. The organic layer is washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the solution is tested for (S)-tert-butyl sulfoxide. Acetyl tert-butyl thioester external standard yield 92% (169.8g, 0.87mol), 96.5% ee;
References:
Shanghai Hanhong Technology Co., Ltd.;Ma Shizhong;Tan Zaipei;Zhang Baohua;Zha Liangxing;Du Xianglong CN106478471, 2017, A Location in patent:Paragraph 0018; 0019

110-06-5
180 suppliers
$11.00/25g
67734-35-4
85 suppliers
$45.00/50mg

60011-16-7
72 suppliers
$41.00/250mg