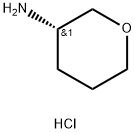
(S)-Tetrahydro-2H-pyran-3-amine hydrochloride synthesis
- Product Name:(S)-Tetrahydro-2H-pyran-3-amine hydrochloride
- CAS Number:1245724-46-2
- Molecular formula:C5H12ClNO
- Molecular Weight:137.61
![Carbamic acid, N-[(3S)-tetrahydro-2H-pyran-3-yl]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/1295722-66-5.gif)
1295722-66-5

1245724-46-2
General procedure for the synthesis of (S)-3-aminotetrahydropyran hydrochloride from the compound (CAS: 1295722-66-5): the intermediate (S)-tert-butyltetrahydro-2H-pyran-3-ylcarbamate (414 g, 2.06 mol) was added to a methanolic solution (4 L) of 6 N HCl, and the reaction mixture was stirred at room temperature until the reaction was complete (monitored by TLC monitoring). Upon completion of the reaction, the mixture was concentrated under vacuum to afford 283 g of (S)-(tetrahydropyran-3-yl)amine hydrochloride as a white solid (99.8% yield, enantiomeric excess >99%). The structure of the product was confirmed by 1H NMR (400 MHz, DMSO-d6): δ 8.37 (s, 3H), 3.87-3.72 (m, 1H), 3.70-3.57 (m, 1H), 3.54-3.38 (m, 2H), 3.12 (d, J = 2.1 Hz, 1H), 2.05-1.89 (m, 1H), 1.81 -1.60 (m, 2H), 1.49 (dtd, J = 12.5, 8.3, 4.3 Hz, 1H).
![Carbamic acid, N-[(3S)-tetrahydro-2H-pyran-3-yl]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/1295722-66-5.gif)
1295722-66-5
0 suppliers
inquiry

1245724-46-2
76 suppliers
inquiry
Yield:1245724-46-2 99.8%
Reaction Conditions:
with hydrogenchloride in methanol at 20;
Steps:
1
(S)-(Tetrahydro-pyran-3-yl)amine hydrochloride Intermediate (S)-tert-butyl tetrahydro-2H-pyran-3-ylcarbamate (414 g, 2.06 mol) was added to a 6N HC1 solution of MeOH (4 L) at room temperature, and the reaction mixture was stirred until it was complete (monitored by TLC). After concentration in vacuo, 283 g of (S)- (tetrahydro-pyran-3-yl)amine hydrochloride was obtained as a white solid (yield, 99.8%, enantiomeric excess >99%). 1H NMR (400 MHz, DMSO-d6) δ 8.37 (s, 3H), 3.87 - 3.72 (m, 1H), 3.70 - 3.57 (m, 1H), 3.54 - 3.38 (m, 2H), 3.12 (d, J = 2.1 Hz, 1H), 2.05 - 1.89 (m, 1H), 1.81 - 1.60 (m, 2H), 1.49 (dtd, J= 12.5, 8.3, 4.3 Hz, 1H).
References:
WO2013/7768,2013,A1 Location in patent:Page/Page column 110; 111
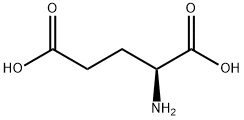
56-86-0
872 suppliers
$5.00/25g

1245724-46-2
76 suppliers
inquiry
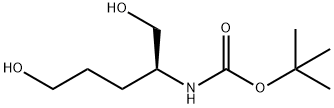
162955-48-8
62 suppliers
$57.00/1/ G

1245724-46-2
76 suppliers
inquiry