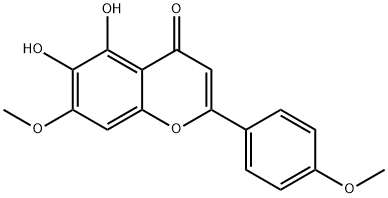
Scutellarein 4',7- dimethyl ether synthesis
- Product Name:Scutellarein 4',7- dimethyl ether
- CAS Number:10176-71-3
- Molecular formula:C17H14O6
- Molecular Weight:314.29
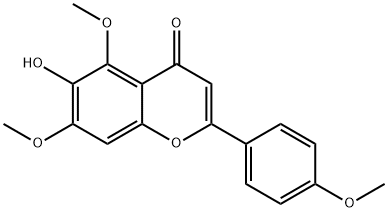
6938-19-8
3 suppliers
inquiry

10176-71-3
34 suppliers
inquiry
Yield: 82%
Reaction Conditions:
Stage #1:6-hydroxy-5,7-dimethoxy-2-(4-methoxyphenyl)-4H-chromen-4-one with boron tribromide in dichloromethane at 0; for 0.5 h;
Stage #2: with methanol
Steps:
Synthesis of Test Compounds
The synthesis of flavone 13 and of its fluorine analogues 14, 15 was executed in a straightforward manner as outlined in Reaction Scheme 1. The commercially available 4,6-dimethoxy-2-hydroxy acetophenone was subjected to Elbs oxidation (Elbs, 1893) using sodium persulfate and aqueous sodium hydroxide to afford the substituted acetophenone 1 in yield between 31 - 64 %. The reaction conditions were applied according to reported procedure but with slight modifications. The yield of the first step is significantly increased upon addition of solid potassium persulfate to the 2-days-old reaction mixture and upon several acidic treatments and heating of the reaction mixture. The generation of the trilithium trianion 2 of the acetophenone 1 was produced by treatment with LiHMDS (Lithium hexamethyldisilylazide). Treatment of trilithium trianion 2 with commercially available benzoyl chloride (3, 4, 5), followed by acidification, afforded the diketone intermediates (6, 7, 8), which were used without further purification for cyclization. The crude intermediates 6, 7, 8 were heated at 95 - 100 °C in the presence of glacial acetic acid containing 0.5% sulfuric acid for 3.5 h to provide the flavone 9 (in the case of 6) and 6h to provide flavones 11, 12 (in the case of 7, 8, respectively). Hydrolysis of the ester function by lithium hydroxide afforded the flavone 10. Thus, a short and economical synthesis of the flavone 10 was performed in 56% overall yield following a 4 steps-sequence from the acetophenone 1. In the case of fluorine analogues the cyclization and the cleavage of the ester group was observed in one step. Final demethylation of flavone 10 was achieved in the presence of boron tribromide affording the flavone 13 (herein also referred to as flavone 13, compound 13 or BJ486K; notably, BJ486K is the synthetic counterpart of the structurally identical flavonoid MP03 (also known as ladanein) isolated from plant extracts of Marrubium peregrinum Lamiaceae) after hydrolysis by methanol in high 82% yield as well as the demethylation step of flavones 11, 12 to afford 14 and 15 in 93% yield and 27% yield, respectively. Purification of final very pure flavones was performed by low pressure column chromatographic techniques (Sephadex LH20 and CH2C12/CH3OH gradients) under nitrogen, and the structures elucidated by NMR and ESI-MS. A mixture of water (20 mL), dibromomethane(150 mmole) and Adogen 464 (1 mnole) was rigorously stirred and heated to reflux. The air in the system was displaced by Argon. A solution of the appropriate catecholflavones 13-15 (100 mmole) and sodium hydroxide (250 mmole) in water (50 mL) was added at such a rate that the addition was complete after 2 hours. After the addition was complete, the reaction mixture was stirred and refluxed for a further hour. The product 16-18 were then isolated by column chromatographic techniques and identified by NMR and ESI-MS.
References:
Twincore Zentrum für Experimentelle und Klinische Infektionsforschung GmbH;CNRS Centre National de la Recherche Scientifique;Université de Strasbourg;Pietschmann, Thomas;Haid, Sibylle;Gentzsch, Juliane;Grethe, Christina;Davioud-Charvet, Elisabeth;Lanfranchi, Don Antoine;Elhabiri, Mourad;Benlloch-Martin, Xavier EP2641904, 2013, A1 Location in patent:Paragraph 0091-0092
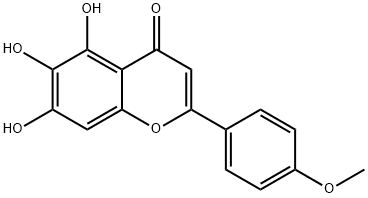
6563-66-2
10 suppliers
$343.00/1mg

74-88-4
356 suppliers
$15.00/10g

10176-71-3
34 suppliers
inquiry
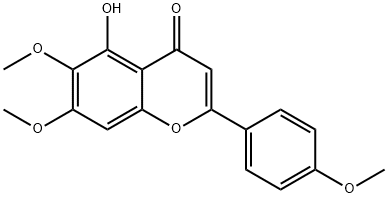
19103-54-9
97 suppliers
$50.00/1 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

10176-71-3
34 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

10176-71-3
34 suppliers
inquiry
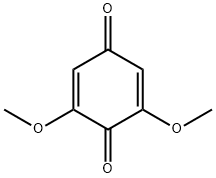
530-55-2
147 suppliers
$23.50/1g

10176-71-3
34 suppliers
inquiry