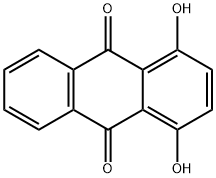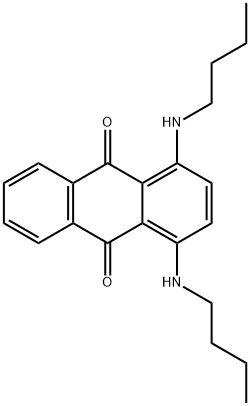
Solvent Blue 35 synthesis
- Product Name:Solvent Blue 35
- CAS Number:17354-14-2
- Molecular formula:C22H26N2O2
- Molecular Weight:350.45
Yield:17354-14-2 258.2 mg
Reaction Conditions:
with pyridine at 50; for 120 h;
Steps:
3.5. General procedure for butylamination of 1-p-toluonesulfonyloxy-2-alkyl-9,10-anthraquinones (3a-c), 1-p-toluonesulfonyloxy-9,10-anthraquinone (3d), 1,4-bis(p-toluonesulfonyloxy)-2-alkyl-9,10-anthraquinones (3a' -c’) and 1,4- bis(p-toluonesulfonyloxy)-9,10-anthraquinone (3d' ) (reaction III, Fig. 1)
General procedure: A large excess (400-1000 mol eq.) of n-butylamine was added to asolution of the respective tosylated compound in pyridine and the reactionmixture was kept at 50 C for 2-5 days to ensure completion ofthe reaction which was monitored by TLC (SiO2; heptane-ethylacetateratio 6:1). After cooling, pyridine was removed by co-evaporation with toluene. Column chromatography was then performed (SiO2,heptane-to-ethylacetate ratio 6:1), the first eluting red (for educts 3a-d)and blue fraction (for educts 3a-d’), respectively, was collected. Afterevaporation of solvent, the obtained solids were recrystallized fromabsolute ethanol.
References:
Jaxel, Julien;Amer, Hassan;Bacher, Markus;Roller, Alexander;Guggenberger, Matthias;Zwirchmayr, Nele Sophie;Hansmann, Christian;Liebner, Falk [Dyes and Pigments,2020,vol. 173,art. no. 107991]
6119-74-0
6 suppliers
inquiry

109-73-9
461 suppliers
$9.00/1g

17354-14-2
242 suppliers
$5.00/25g
82874-66-6
0 suppliers
inquiry
28736-42-7
97 suppliers
$36.00/250mg

109-73-9
461 suppliers
$9.00/1g

17354-14-2
242 suppliers
$5.00/25g
![9,10-Anthracenedione, 1,4-bis[[(4-methylphenyl)sulfonyl]oxy]-](/CAS/20210305/GIF/96701-06-3.gif)