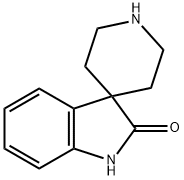
SPIRO[INDOLINE-3,4'-PIPERIDIN]-2-ONE synthesis
- Product Name:SPIRO[INDOLINE-3,4'-PIPERIDIN]-2-ONE
- CAS Number:252882-61-4
- Molecular formula:C12H14N2O
- Molecular Weight:202.25
![1'-benzylspiro[indoline-3,4'-piperidin]-2-one](/CAS/20200331/GIF/1086063-19-5.gif)
1086063-19-5
![SPIRO[INDOLINE-3,4'-PIPERIDIN]-2-ONE](/CAS/GIF/252882-61-4.gif)
252882-61-4
Method 1: Procedure for the synthesis of spiro[indolin-3,4'-piperidin]-2-one. Hydroxyindole (3.00 g, 22.5 mmol) was dissolved in tetrahydrofuran (20 mL) under argon protection, and a tetrahydrofuran solution (20 mL) with benzylbis(2-chloroethyl)amine (5.20 g, 22.5 mmol) was added sequentially to tetrahydrofuran (100 mL). Sodium hydride (1.60 g, 67.6 mmol) was added at room temperature and the mixture was stirred at the same temperature for 1 hour, then warmed up to 90 °C and continued stirring for 3 hours. After the reaction solution was cooled to room temperature, a tetrahydrofuran solution (10 mL) of sodium hydride (0.540 g, 22.5 mmol) was added and stirring was continued at 90 °C for 12 hours. After the reaction was completed, saturated aqueous ammonium chloride solution was added and stirred for 10 min at room temperature. The mixture was poured into a mixture of saturated aqueous sodium bicarbonate solution and brine and extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: chloroform:methanol=20:1) to afford 1'-benzylspiro[indoline-3,4'-piperidin]-2-one (2.94 g, 44.6% yield) as a yellow amorphous solid.1H-NMR (400 MHz, CDCl3) δ: 1.78-1.93 (m, 2H), 1.95-2.06 (m, 2H), 2.65-2.06 (m, 2H). 2H), 2.65-2.77 (m, 2H), 2.87-3.00 (m, 2H), 3.69 (s, 2H), 6.90 (d, J=7.6Hz, 1H), 7.02 (t, J=7.6Hz, 1H), 7.20 (t, J=7.6Hz, 1H), 7.26 (t, J=6.2Hz, 1H), 7.34 (t, J=6.2Hz, 1H), 7.34 (t, J=7.6Hz, 1H). J=7.6 Hz, 2H), 7.40-7.41 (m, 3H), 8.72 (s, 1H). 1'-Benzylspiro[indoline-3,4'-piperidin]-2-one (300 mg, 1.03 mmol) was dissolved in methanol (5 mL) and 10% palladium carbon (30.0 mg) was added. The mixture was stirred at room temperature under hydrogen atmosphere for 15 hours. The reaction solution was filtered through diatomaceous earth and concentrated under reduced pressure to give spiro[indoline-3,4'-piperidin]-2-one (187 mg, 90.2% yield) as a colorless amorphous solid.1H-NMR (400 MHz, CDCl3) δ: 1.73-1.78 (m, 2H), 1.88-1.94 (m, 2H), 3.06-3.12 (m, 2H) , 3.35-3.41 (m, 2H), 6.92 (d, J=7.7Hz, 1H), 7.04 (t, J=7.7Hz, 1H), 7.22 (t, J=7.7Hz, 1H), 7.42 (d, J=7.7Hz, 1H), 8.70 (br, 1H).
![1'-benzylspiro[indoline-3,4'-piperidin]-2-one](/CAS/20200331/GIF/1086063-19-5.gif)
1086063-19-5
8 suppliers
inquiry
![SPIRO[INDOLINE-3,4'-PIPERIDIN]-2-ONE](/CAS/GIF/252882-61-4.gif)
252882-61-4
61 suppliers
$59.00/100mg
Yield:252882-61-4 90.2%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol at 20; for 15 h;
Steps:
1.1
Process 1Production of spiro(indole-3,4'-piperidine)-2(1H)-oneSpiro(indole-3,4'-piperidine)-2(1H)-one was produced by the method described below.Under an argon atmosphere, a tetrahydrofuran solution (20 mL) of oxindole (3.00 g, 22.5 mmol) and a tetrahydrofuran solution (20 mL) of benzylbis(2-chloroethyl)amine (5.20 g, 22.5 mmol) were added sequentially to a tetrahydrofuran solution (100 mL) of sodium hydride (1.60 g, 67.6 mmol) at room temperature. The mixture was stirred at the same temperature for 1 hour and further stirred at 90° C. for 3 hours. The reaction solution was cooled to room temperature, and then added with a tetrahydrofuran solution (10 mL) of sodium hydride (0.540 g, 22.5 mmol) and stirred further at 90° C. for 12 hours. The reaction solution was added with saturated ammonium-chloride aqueous solution and stirred at room temperature for 10 minutes. The mixed solution was poured into the mixed solution of a saturated aqueous solution of sodium hydrogen carbonate and brine, then extracted with ethyl acetate. The organic layer was dried with anhydrous sodium sulfate, followed by a vacuum concentration. The resultant residue was purified by silica-gel chromatography (chloroform:methanol=20:1) and 1'-benzylspiro(indole-3,4'-piperidine)-2(1H)-one (2.94 g, 44.6%) was obtained as a yellow amorphous solid.1H-NMR (400 MHz, CDCl3) δ; 1.78-1.93 (m, 2H), 1.95-2.06 (m, 2H), 2.65-2.77 (m, 2H), 2.87-3.00 (m, 2H), 3.69 (s, 2H), 6.90 (d, J=7.6 Hz, 1H), 7.02(t, J=7.6 Hz, 1H), 7.20 (t, J=7.6 Hz, 1H), 7.26 (t, J=6.2 Hz, 1H), 7.34 (t, J=7.6 Hz, 2H), 7.40-7.41(m, 3H), 8.72 (s, 1H).To a methanol solution (5 mL) of 1'-benzylspiro(indole-3,4'-piperidine)-2(1H)-one (300 mg, 1.03 mmol), 10% palladium carbon (30.0 mg) was added. The mixture was stirred under a hydrogen atmosphere at room temperature for 15 hours. The reaction solution was filtered using celite followed by a vacuum concentration, and spiro(indole-3,4'-piperidine)-2(1H)-one (187 mg, 90.2%) was obtained as a colorless amorphous solid.1H-NMR (400 MHz, CDCl3) δ; 1.73-1.78 (m, 2H), 1.88-1.94 (m, 2H), 3.06-3.12 (m, 2H), 3.35-3.41 (m, 2H), 6.92 (d, J=7.7 Hz, 1H), 7.04 (t, J=7.7 Hz, 1H), 7.22 (t, J=7.7 Hz, 1H), 7.42 (d, J=7.7 Hz, 1H), 8.70 (br, 1H).
References:
US2008/306102,2008,A1 Location in patent:Page/Page column 12
![1,2-DIHYDRO-2-OXO-SPIRO[3H-INDOLE-3,4'-PIPERIDINE]-1'-CARBOXYLIC ACID 1,1-DIMETHYLETHYL ESTER](/CAS/GIF/252882-60-3.gif)
252882-60-3
103 suppliers
$30.00/100mg
![SPIRO[INDOLINE-3,4'-PIPERIDIN]-2-ONE](/CAS/GIF/252882-61-4.gif)
252882-61-4
61 suppliers
$59.00/100mg
59-48-3
478 suppliers
$5.00/1g
![SPIRO[INDOLINE-3,4'-PIPERIDIN]-2-ONE](/CAS/GIF/252882-61-4.gif)
252882-61-4
61 suppliers
$59.00/100mg