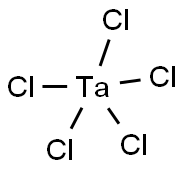
Tantalum(V) chloride synthesis
- Product Name:Tantalum(V) chloride
- CAS Number:7721-01-9
- Molecular formula:Cl5Ta
- Molecular Weight:358.21
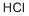
7647-01-0
0 suppliers
$10.00/10g

7721-01-9
153 suppliers
$28.00/5G
Yield:-
Reaction Conditions:
with Ta-oxyd hydrate in meltin KCl-NaCl-melt; no pure prod.;
References:
Horizons Titanium Corp. US2870073, 1959, A [C.A.,1959,p. 5926][C.A.,1959,p. 5926]