tert-butyl 3-oxocycloheptylcarbamate synthesis
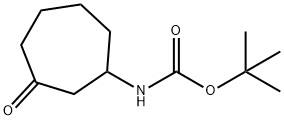
- Product Name:tert-butyl 3-oxocycloheptylcarbamate
- CAS Number:1209481-80-0
- Molecular formula:C12H21NO3
- Molecular Weight:227.3
Yield:1209481-80-0 61%
Reaction Conditions:
with bismuth(III) nitrate in dichloromethane at 20; for 24 h;
Steps:
134.1 Step 1: Synthesis of tert-butyl N-(3-oxocycloheptyl)carbamate
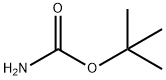
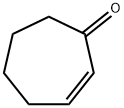
To a solution of cyclohept-2-en-1-one (6.00 g, 54.47 mmol) and tert-butyl carbamate (6.38 g, 54.47 mmol) in dichloromethane (240.0 mL) was added bismuth nitrate (10.76 g, 27.23 mmol) in portions. After stirring at 20 °C for 24 hours, the reaction mixture was diluted with dichloromethane and washed with brine. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. Purification via silica gel column chromatography (ethyl acetate/petroleum ether) afforded the title compound as a light yellow solid. Yield: 9.00 g, 61%.1H NMR (Chloroform-d, 400 MHz) δ 4.58 (s, 1H), 3.91 (s, 1H), 2.78- 2.86 (m, 1H), 2.43-2.65 (m, 3H), 1.91-2.00 (m, 1H), 1.79-1.89 (m, 1H), 1.60-1.77 (m, 4H), 1.43 (s, 9H).
References:
WO2022/109492,2022,A1 Location in patent:Page/Page column 454-455