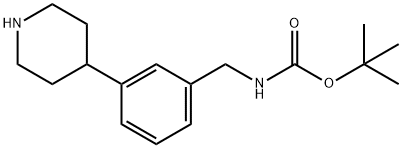
tert-butyl 3-(piperidin-4-yl)benzylcarbaMate synthesis
- Product Name:tert-butyl 3-(piperidin-4-yl)benzylcarbaMate
- CAS Number:725228-49-9
- Molecular formula:C17H26N2O2
- Molecular Weight:290.4
![Carbamic acid, N-[[3-(4-piperidinyl)phenyl]methyl]-, 1,1-dimethylethyl ester, acetate (1:1)](/CAS/20211123/GIF/864069-14-7.gif)
864069-14-7
0 suppliers
inquiry

725228-49-9
15 suppliers
inquiry
Yield:725228-49-9 86%
Reaction Conditions:
Stage #1: 4-[3-(tert-butoxycarbonylaminomethyl)phenyl]piperidine acetic acid saltwith hydrogenchloride in water;
Stage #2: with sodium hydroxide in water;
Steps:
1.d
d) 4-[3-(tert-Butoxycarbonylaminomethyl)phenyl]piperidine 4-[3-(tert-Butoxycarbonylaminomethyl)phenyl]piperidine acetic acid salt (147 g, 0.42 mol) is dissolved in dilute hydrochloric acid and the solution extracted with ether. The aqueous phase is basified with sodium hydroxide and extracted with ether. The organic phase is concentrated in vacuo and the product is precipitated from pentane/ether yielding the title compound (105 g, 86%) as an off-white solid. 1H NMR (300 MHz, CDCl3) δ = 7.25 (m, 1 H), 7.07-7.13 (m, 3 H), 5.12 (m, 1 H), 4.29 (d, 2 H), 3.17 (m, 3 H), 2.72 (m, 2 H), 2.60 (m, 1 H), 1.81 (m, 2 H), 1.55-1.70 (m, 2 H), 1.46 (s, 9 H). MS (ESI) m/z 291 (M+ +1, 100).
References:
EP1571150,2005,A1 Location in patent:Page/Page column 37
![Carbamic acid, [[3-(4-piperidinyl)phenyl]methyl]-, 1,1-dimethylethyl ester, mono(4-methylbenzenesulfonate) (9CI)](/CAS/20211123/GIF/864069-18-1.gif)
864069-18-1
0 suppliers
inquiry

725228-49-9
15 suppliers
inquiry

375854-52-7
10 suppliers
inquiry

725228-49-9
15 suppliers
inquiry
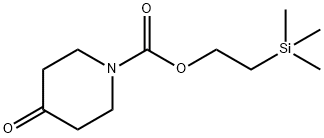
181701-30-4
8 suppliers
$834.00/5g

725228-49-9
15 suppliers
inquiry
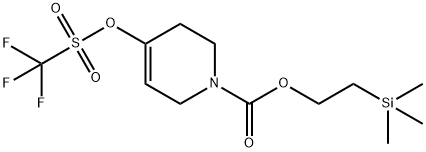
375854-77-6
20 suppliers
$137.00/100mg

725228-49-9
15 suppliers
inquiry