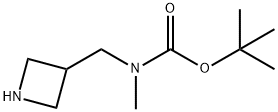
tert-butyl azetidin-3-ylmethyl(methyl)carbamate synthesis
- Product Name:tert-butyl azetidin-3-ylmethyl(methyl)carbamate
- CAS Number:1053655-53-0
- Molecular formula:C10H20N2O2
- Molecular Weight:200.28

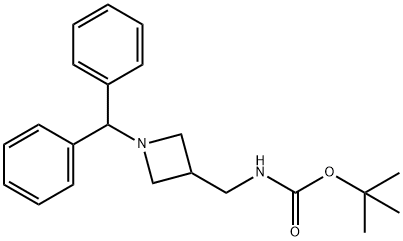
91189-19-4
32 suppliers
$131.59/1g

1053655-53-0
47 suppliers
$45.00/10mg
Yield:-
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol;water;ethyl acetate for 18 h;Inert atmosphere;
Steps:
2
A solution of the compound obtained above (6.18 g, 17.53 mmol) in 50 mL of MeOH and 15 mL of EtOAc was purged with argon. Pd/C (10%, 50% in water) (929 mg) was added and the mixture was then purged again with argon and stirred in a H2 atmosphere for 18 hours. The reaction was filtered through Ceitte and the filter was washed with EtOAc and MeOH, The solvent was evaporated to dryness, providing 5.66 g of a mixture of the title compound together with one equivalent of diphenylmethane, that was used as such in the following steps.iH NMR (300 MHz, CD3OD) δ: 1.44 (s, 9H), 2.88 (s, 3H), 3.56 (m, 2H), 3.71 (m, 2H), 4.75 (m, 1H).
References:
Location in patent:Page/Page column 52

1064048-70-9
11 suppliers
inquiry

1053655-53-0
47 suppliers
$45.00/10mg