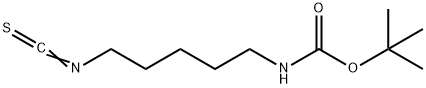
TERT-BUTYL N-(5-ISOTHIOCYANATOPENTYL)CARBAMATE synthesis
- Product Name:TERT-BUTYL N-(5-ISOTHIOCYANATOPENTYL)CARBAMATE
- CAS Number:347890-46-4
- Molecular formula:C11H20N2O2S
- Molecular Weight:244.35
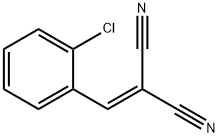
2698-41-1
137 suppliers
inquiry
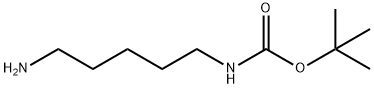
51644-96-3
178 suppliers
$23.00/1g

347890-46-4
19 suppliers
$67.29/100MG
Yield:347890-46-4 93%
Reaction Conditions:
Stage #1: o-chlorobenzylidene malononitrile;5-tert-butoxycarbonylamino-1-aminopentane in tetrahydrofuran at 20; for 14 h;
Stage #2: with CYANAMID;triethylamine at 4; for 3 h;
Steps:
AD Example AD
(5-Isothiocyanato-pentyl)-carbamic Acid tert-butyl Ester
To a solution of 2 g (9.9 mmol) (5-Amino-pentyl)-carbamic acid tert-butyl ester in 40 ml THF at 0° C. was added 896 μl (14.83 mmol) CS2 and allowed to stir at room temperature for 14 h. 623 mg (14.83 mmol) cyanamide and 4 drops NEt3 was added and the mixture was heated to 4° C. for 3 h.The mixture was extracted with diethyl ether and the combined organic layers were dried with MgSO4.After filtration and removal of the volatiles the residue was purified by flash column chromatography on silica eluding with ethyl acetate/cyclohexane 1:1.The evaporation of the product fractions yielded 2.24 g (93%) of the title compound. 1-H-NMR (250 MHz, CDCl3) δ=4.58 (s, br, 1H, NH), 3.52 (t, J=6.5 Hz, 2H, NCH2), 3.13 (dd, J1=6.5 Hz, J2=4 Hz, 2H, NHC2), 1.74 (m, 2H, CH2), 1.50 (m, 4H, CH2), 1.44 (s, 9H, CH3). MS (m/e): 262.3 (M+NH4, 100%)
References:
US2003/225141,2003,A1 Location in patent:Page 27