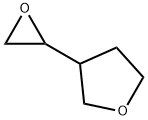
tetrahydro-3-(2-oxiranyl)Furan synthesis
- Product Name:tetrahydro-3-(2-oxiranyl)Furan
- CAS Number:1425972-13-9
- Molecular formula:C6H10O2
- Molecular Weight:114.14
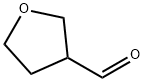
79710-86-4
94 suppliers
$15.00/1g
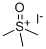
1774-47-6
557 suppliers
$6.00/25g

1425972-13-9
10 suppliers
inquiry
Yield:1425972-13-9 14%
Reaction Conditions:
Stage #1: trimethylsulfoxonium iodidewith potassium tert-butylate in 1,2-dimethoxyethane at 0 - 20; for 0.5 h;
Stage #2: tetrahydrofuran-3-carboxaldehyde in 1,2-dimethoxyethane; for 1 h;
Steps:
2.139.a Step a Intermediate 173 -(oxiran-2-yl)tetrahydrofuran
Step a Intermediate 173 -(oxiran-2-yl)tetrahydrofuran To a suspension of trimethylsulfoxonium iodide (2.1 g, 9.4 mmol) in DME (20 ml) was added O'Bu (1.15 g, 9.4 mmol). The reaction was stirred for 30 min at r.t. and was then cooled to 0 °C. A solution of tetrahydrofuran-3-carbaldehyde (858 mg, 8.6 mmol) in DME was added dropwise and the reaction stirred for 1 h. The reaction was quenched via the addition of sat. aq. NH4C1 and extracted with EtOAc. The organics were washed with sat. aq. NaHC03 and brine then dried (MgS04) and concentrated under reduced pressure to give 3-(oxiran-2-yl)tetrahydrofuran (140 mg, 14 %). ? NMR (300MHz, CDC13) δ 3.70 (m, 4H), 2.92 (m, 1H), 2.80 (q, 1H), 2.53 (m, 1H), 1.80 (m, 3H)
References:
WO2013/27001,2013,A1 Location in patent:Page/Page column 150