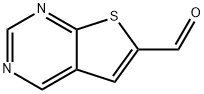
Thieno[2,3-d]pyrimidine-6-carbaldehyde synthesis
- Product Name:Thieno[2,3-d]pyrimidine-6-carbaldehyde
- CAS Number:655253-72-8
- Molecular formula:C7H4N2OS
- Molecular Weight:164.18
![Thieno[2,3-d]pyrimidine-6-carboxamide, N-methoxy-N-methyl-](/CAS/20210305/GIF/655253-71-7.gif)
655253-71-7
0 suppliers
inquiry
![Thieno[2,3-d]pyrimidine-6-carbaldehyde](/CAS/20200401/GIF/655253-72-8.gif)
655253-72-8
3 suppliers
inquiry
Yield:655253-72-8 70%
Reaction Conditions:
Stage #1: N-methoxy-N-methylthieno[2,3-d]pyrimidine-6-carboxamidewith lithium aluminium tetrahydride in tetrahydrofuran at -78 - 0; for 0.5 h;
Stage #2: with water;ammonium chloride in tetrahydrofuran at 0; for 0.166667 h;
Steps:
13 Intermediate 13; [THIENOR2, 3-DLPVRIMIDINE-6-CARBALDEHVDE]
Lia1H4 [(1M] in THF, 0.26 rnL) was added dropwise to N-methoxy-N- methylthieno [2,3-d] pyrimidine-6-carboxamide (intermediate 12) (117 mg) in THF [(6] mL) at -78°C under an inert atmosphere. The reaction was allowed to stir at-78 °C for 30 minutes and then allowed to warm to [0 °C.] Saturated aqueous ammonium chloride (2 mL) was added and allowed to stir at [0 °C] for 10 minutes. The organic layer was separated and the aqueous layer extracted with diethyl ether (2 x 15 mL). The combined organics were dried [(MGS04),] filtered and concentrated in vacuo. Purification by flash chromatography on silica eluting with hexane: EtOAc (1: 4) afforded the title compound as a white solid (60 mg, 70%); [1H NMR (CDCL3) No. ] 8.06 (s, 1H), 9.22 (s, 1H), 9.33 (s, 1H), 10.15 (s, 1H); [MS M/E I+] 165.
References:
WO2004/13141,2004,A1 Location in patent:Page 89