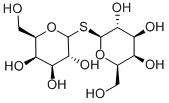
THIODIGALACTOSIDE synthesis
- Product Name:THIODIGALACTOSIDE
- CAS Number:51555-87-4
- Molecular formula:C12H22O10S
- Molecular Weight:358.36

18968-44-0
0 suppliers
inquiry

51555-87-4
75 suppliers
$39.00/25mg
Yield:-
Reaction Conditions:
with methanol;sodium methylate at 20;
Steps:
4.1.4. General procedure for zemplen deacetylation
General procedure: To a solution of peracetylated diglycoside sulfide (0.2-0.4 mmol) inMeOH was added a solution of sodium methoxide in methanol (0.1 eq).The mixture was left under stirring at rt until detection via TLC (eluent:ethyl acetate) of the reaction completion (generally reactions took2-4 h). The mixture was treated with Amberlyst H+ resin until neutrality,and supernatant liquid was transferred via pipette to anothervessel. Resin was washed with further methanol which was thentransferred to the same vessel. Removal of methanol in vacuo provideddeprotected sulfides. β-D-galactopyranosyl 1-thio-β-D-galactopyranoside (1):1H NMR(400 MHz, D2O) δ 4.86 (d, 1H, J=9.6 Hz, H-1), 4.04 (d, 1H,J=3.2 Hz, H-4), 3.90-3.75 (overlapped signals, 3H, H-5 and H2-6),3.74 (dd, 1H, J=3.2 and 9.6 Hz, H-3), 3.68 (t, 1H, J=9.6 Hz, H-2).13C NMR (100 MHz, D2O) δ 83.5 (C-1), 79.0, 73.9, 69.6, 68.8, 61.2.Anal. Calcd. for C12H22O10S: C, 40.22; H, 6.19. Found: C, 40.10; H,6.25. MALDI HRMS m/z [M + Na]+ calc. for (C12H22O10SNa)381.0831, found 381.0835.
References:
Di Gaetano, Sonia;Bedini, Emiliano;Landolfi, Alfredo;Pedone, Emilia;Pirone, Luciano;Saviano, Michele;Traboni, Serena;Capasso, Domenica;Iadonisi, Alfonso [Carbohydrate Research,2019,vol. 482,art. no. 107740]