
Thiosalicylic acid synthesis
- Product Name:Thiosalicylic acid
- CAS Number:7283-41-2
- Molecular formula:C7H6O2S
- Molecular Weight:154.19
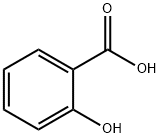
69-72-7
1314 suppliers
$5.00/10g

7283-41-2
3 suppliers
inquiry
Yield:-
Reaction Conditions:
with Lawessons reagent in acetonitrile at 100; for 0.25 h;Inert atmosphere;Microwave irradiation;
Steps:
4.1.2 Procedure B: general procedure for synthesis of thio-acids
General procedure: To a solution of substituted aromatic acid (1 eq.) in dry acetonitrile was added Lawesson's reagent (0.5 eq.) and was subjected to microwave irradiation in closed vessel at 100°C for 15min (Antonpaar monowave 300 instrument). The reaction mixture was evaporated to dryness and the crude residue obtained was diluted with EtOAc and washed several times with 1N HCl, then with brine solution and dried over anhydrous Na2SO4. The combined organic layers were concentrated in vacuo and purified by silica gel column chromatography with elution of 20-30% EtOAc/Petroleum ether. * All thio-acids are prepared freshly before reaction and used immediately for reactions.
References:
Philkhana, Satish Chandra;Verma, Abhishek Kumar;Jachak, Gorakhnath R.;Hazra, Bibhabasu;Basu, Anirban;Reddy, D. Srinivasa [European Journal of Medicinal Chemistry,2017,vol. 135,p. 89 - 109]