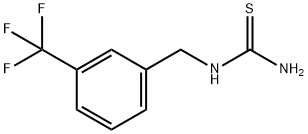
Thiourea, N-[[3-(trifluoromethyl)phenyl]methyl]- synthesis
- Product Name:Thiourea, N-[[3-(trifluoromethyl)phenyl]methyl]-
- CAS Number:296277-06-0
- Molecular formula:C9H9F3N2S
- Molecular Weight:234.24
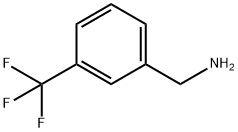
2740-83-2
189 suppliers
$10.00/1g
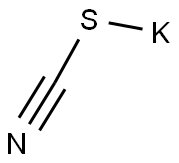
333-20-0
464 suppliers
$13.50/100G
![Thiourea, N-[[3-(trifluoromethyl)phenyl]methyl]-](/CAS/20210111/GIF/296277-06-0.gif)
296277-06-0
1 suppliers
inquiry
Yield:296277-06-0 12%
Reaction Conditions:
Stage #1: 3-(TRIFLUOROMETHYL)BENZYLAMINEwith hydrogenchloride in water;Cooling with ice;
Stage #2: potassium thioacyanate in tetrahydrofuran;water at 80; for 24 h;
Steps:
General procedure of the synthesis of benzylthiourea derivatives (2a-2g)
General procedure: Benzylamine was added to the reaction flask in ice bath. Thenc-HCl was slowly added to afford a salt. This salt was dissolved in THF then KSCN (1.5 equiv) was added. The reaction mixture was stirred at 80 °C for 24 h.The reaction progress was monitored by TLC and the solvent was removed invacuoand then added H2O, extracted with EtOAc, the organic layer was dried over MgSO4, filtered, concentrated invacuo. The residue was purified by silica gel column chromatography (17% EtOAc/hexanes) to afford desired product.
References:
Jo, Hyeju;Zhou, Yuanyuan;Viji, Mayavan;Choi, Minho;Lim, Jae Young;Sim, Jaeuk;Rhee, Jeongtae;Kim, Youngsoo;Seo, Seung-Yong;Kim, Wun-Jae;Hong, Jin Tae;Lee, Heesoon;Lee, Kiho;Jung, Jae-Kyung [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 21,p. 4854 - 4857] Location in patent:supporting information