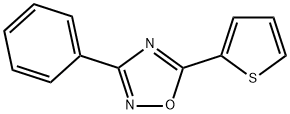
tioxazafen synthesis
- Product Name:tioxazafen
- CAS Number:330459-31-9
- Molecular formula:C12H8N2OS
- Molecular Weight:228.27
5271-67-0
264 suppliers
$5.00/1g
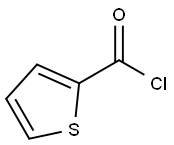
613-92-3
194 suppliers
$5.00/250mg

330459-31-9
34 suppliers
inquiry
Yield: 95%
Reaction Conditions:
with tetra(n-butyl)ammonium hydroxide;sodium hydroxide in 2-methyltetrahydrofuran;water at 70; for 1.33333 h;Solvent;Temperature;Time;Reagent/catalyst;
Steps:
2 Example 2: Preparation of 3-Phenyl-5-(thiophen-2-yl)-l,2,4-oxadiazole in 2-Methyl- tetrahydrofuran
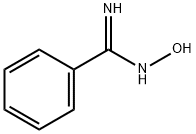
A 39.45% solution of benzamide oxime in 2-methyltetrahydrofuran (33.34 g) was charged to a 100 mL flask, followed by water (9.0 g), 40% aqueous solution of tetrabutylammonium hydroxide (1.02g), and 51% sodium hydroxide (3.52 g, 0.045 moles). To this mixture was added 2-thiophenecarbonyl chloride (13.83 g, 0.0936 moles) and 51% sodium hydroxide (7.33 g, 0.0935 moles) simultaneously over a 30 minute period while heating to about 70°C. The reaction was complete in less than 80 minutes. The aqueous salt phase was removed, water was added (30 g), and the 2-methyltetrahydrofuran partly removed by atmospheric distillation (13.5 mL). An additional amount (32 g) of hot water was added, and the remainder of the 2-methyltetrahydrofuran was distilled out, during which time the product, 3-phenyl-5-(2- thienyl)-l,2,4-oxadiazole, precipitated. The aqueous slurry of 3-phenyl-5-(2-thienyl)- 1,2,4- oxadiazole was filtered, washed with water and dried overnight in a vacuum oven to yield 3- phenyl-5-(2-thienyl)-l,2,4-oxadiazole, 20.47 g, 99.1% pure, 95.0% yield.
References:
MONSANTO TECHNOLOGY LLC;MILLER, William Harold;GRAHAM, Charles Richard;BROWN, David Louis WO2014/8257, 2014, A2 Location in patent:Paragraph 0036
5813-89-8
104 suppliers
$14.00/1g
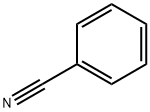
100-47-0
381 suppliers
$14.14/25gm:

330459-31-9
34 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

330459-31-9
34 suppliers
inquiry
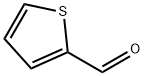
98-03-3
538 suppliers
$5.00/25g

613-92-3
194 suppliers
$5.00/250mg

330459-31-9
34 suppliers
inquiry
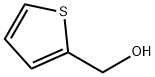
636-72-6
259 suppliers
$6.00/5g