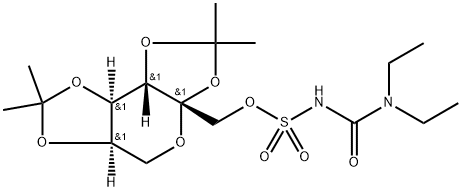
Topiramate Impurity 6 synthesis
- Product Name:Topiramate Impurity 6
- CAS Number:876403-98-4
- Molecular formula:C17H30N2O9S
- Molecular Weight:438.49
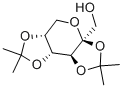
20880-92-6
401 suppliers
$10.00/5g
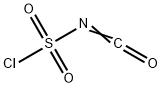
1189-71-5
365 suppliers
$18.00/5g

109-89-7
489 suppliers
$10.00/5g

876403-98-4
30 suppliers
inquiry
Yield: 54%
Reaction Conditions:
Stage #1:isocyanate de chlorosulfonyle;diethylamine in dichloromethane at -25 - 25; for 0.5 h;
Stage #2:2,3;4,5-di-O-isopropylidene-β-D-fructopyranose with triethylamine in dichloromethane at -25 - 25; for 3 h;Product distribution / selectivity;
Steps:
1.2.1; 3.2
A solution of chlorosulfonyl isocyanate (32 g, 0.226 mol) in methylene chloride (100 ml) is cooled to -25° C under an atmosphere of dry argon. Diethylamine (16.52 g, 0.226 mol) in methylene chloride (100 ml) is then added dropwise while the temperature is kept at -20° C. After the addition is finished, the mixture is warmed to 25° C and stirred for 0.5 hours. It is then cooled to -25° C and triethylamine (24.82 g, 0.245 mol) in methylene chloride (40 ml) is added followed by 2,3:4,5-bis-O-(1-methylethylidene)-β-D-fructopyranose (47 g, 0.18 mol) in methylene chloride (60 ml) while the temperature is maintained at -20° C. The resulting mixture is stirred at 25° C and the progress of the reaction is monitored by TLC analysis. After 3 hours completion is reached. Most of the solvent is removed at reduced pressure in a rotary evaporator. The residue is dissolved in methanol (400 ml) and the solution is concentrated to about half its volume in a water bath of 40° C at reduced pressure in a rotary evaporator. The solution is then cooled to 15° C and upon slow addition of water (300 ml) white crystals precipitate. The product is collected by filtration, washed with cold water and dried overnight in vacuum at 40° C. Yield is 56.6 g (0.129 mol, 71.6%). The crude product is recrystallised by dissolving in methanol (150 ml) at 40-45° C and precipitating with water (225 ml) at 15° C. Yield is 42.7 g (0.097 mol, 54%). Melting point (hot plate): 110-112° C [α]20D = -43.7 (c = 1.0, methanol) Characteristic IR bands (cm-1, KBr): 3447, 2989, 2945, 1654, 1477, 1378, 1193, 1159, 1102, 1071, 1058, 1018, 911, 831, 756. 1H-NMR (methanol-d4, 500 MHz) δ: 1.17 (t, J=7 Hz, 6H, N(CH2CH3)2), 1.33, 1.42, 1.44, 1.52 (s each, 12 H overall, CH3 isopropylidene), 3.27 and 3.39 (m each, 4H overall, N(CH2CH3)2), 3.65 (d, 1 H, J=13.0 Hz, H-6a), 3.93 (dd, 1H, J=13 and 1.5 Hz, H-6b), 4.18 (d, 1H, J=10 Hz, H-1 a), 4.24 (d, 2H, J=10 Hz, H-1 b and H-5), 4.49 (d, 1H, J=2.5 Hz, H-3), 4.62 (d, 1 H, J=8 and 2.5 Hz, H-4). 13C-NMR (methanol-d4, 125 MHz) δ: 13.04, (N(CH2CH3)2), 24.46, 25.88, 26.52, 26.91 (CH3 isopropylidene), 42.91 (N(CH2CH3)2), 62.60 (C-6), 71.33 (C-3), 71.54 (C-4), 71.65 (C-1), 72.33 (C-5), 102.20 (C-2), 110.31, 110.62 (Cq isopropylidene), 153.9 (C-O, missing in 1 D spectrum, detectable in gs-HMBC 2D spectrum); Example 3.2 A solution of chlorosulfonyl isocyanate (16.3 g, 0.12 mol) in methylene chloride (50 ml) is cooled to -25° C under an atmosphere of dry argon. Diethylamine (8.49 g, 0.12 mol) in methylene chloride (50 ml) is then added dropwise while the temperature is kept at -20° C. After the addition is finished, the mixture is warmed to 30° C and stirred for 0.5 hours. It is then cooled to -20° C and triethylamine (12.41 g, 0.12 mol) in methylene chloride (10 ml) is added followed by 2,3:4,5-bis-O-(1-methylethylidene)-β-D-fructopyranose (23.1 g, 0.09 mol) in methylene chloride (30 ml) while the temperature is maintained at -15° C. The resulting mixture is stirred at 25° C and the progress of the reaction is monitored by TLC analysis. When completion is reached (3.5-4 hours), sodium acetate-acetic acid buffer (50 ml, pH 3,5) and acetone (10 ml) are added and the mixture is heated to 50° C. After removal of methylene chloride by distillation, further buffer (10 ml) and acetone (10 ml) are added to the reaction mixture and it is refluxed at 76-77°C. After 1.5-2 hours reaction is completed. The mixture is then cooled to room temperature and made alkaline by the addition of 5 M sodium hydroxide. The resulting solution is extracted with tert-butyl methyl ether (3 x 25 ml). Volatiles are removed at reduced pressure and the product crystallises upon neutralisation with 85% phosphoric acid at 5-10° C. The crude product (17.0 g) is recrystallised from 2-propanol (15 ml) and water (50 ml). Yield is 15.3 g (50%).
References:
Helm AG;CF Pharma Gyogyszergyarto Kft. EP1627881, 2006, A1 Location in patent:Page/Page column 9; 11

20880-92-6
401 suppliers
$10.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

876403-98-4
30 suppliers
inquiry