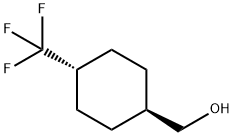
trans-(4-(trifluoromethyl)cyclohexyl)methanol synthesis
- Product Name:trans-(4-(trifluoromethyl)cyclohexyl)methanol
- CAS Number:1202577-61-4
- Molecular formula:C8H13F3O
- Molecular Weight:182.18
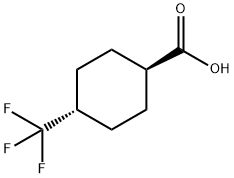
133261-33-3

1202577-61-4
To a suspension of lithium aluminum hydride (11.6 g, 0.306 mol) in anhydrous tetrahydrofuran (350 mL) was added slowly and dropwise anhydrous tetrahydrofuran solution (50 mL) of trans-4-(trifluoromethyl)cyclohexanecarboxylic acid (30 g, 0.153 mol) at 0 °C. The reaction mixture was stirred continuously at 0°C for 2 hours. The progress of the reaction was monitored by thin layer chromatography (TLC, unfolding agent was petroleum ether:ethyl acetate = 10:1) to confirm complete consumption of raw materials. The reaction was quenched sequentially with water (12 mL), 15% aqueous sodium hydroxide solution (24 mL) and water (12 mL). The reaction mixture was filtered and the filtrate was concentrated under reduced pressure to give trans-(4-(trifluoromethyl)cyclohexyl)methanol (24 g, 86% yield) as a colorless liquid. The product was characterized by 1H NMR (CDCl3, 400 MHz): δ 3.49-3.50 (d, J = 6.0 Hz, 2H), 1.91-2.07 (m, 4H), 1.50-1.57 (m, 1H), 1.32-1.36 (m, 2H), 0.98-1.05 (m, 2H).

133261-33-3
103 suppliers
$8.00/100mg

1202577-61-4
50 suppliers
$92.00/100mg
Yield:1202577-61-4 100%
Reaction Conditions:
with borane-THF in tetrahydrofuran at 0 - 20; for 2 h;
Steps:
32 Reference Example 32: Synthesis of trans-4-(trifluoromethyl)cyclohexyl)methanol
trans-4-(Trifluoromethyl)cyclohexane-1-carboxylic acid (0.400 g, 2.04 mmol) was dissolved in THF (5.1 mL) and a solution of borane-THF complex in THF (0.90 M, 2.72 mL, 2.45 mmol) was added at 0° C.
After stirring the reaction mixture at room temperature for 2 hours, distilled water was added to the reaction mixture, the aqueous layer was extracted with ethyl acetate.
The organic layer was washed with brine, dried over anhydrous sodium sulfate and filtered, and then the filtrate was concentrated under reduced pressure to obtain the title compound (hereinafter referred to as the compound of Reference Example 32) (0.370 g, 2.04 mmol, quantitative) as a colorless oil.
1H-NMR (400 MHz, CDCl3) δ: 0.94-1.05 (m, 2H), 1.26-1.38 (m, 3H), 1.43-1.53 (m, 1H), 1.91-2.02 (m, 5H), 3.49 (t, J=5.7 Hz, 2H).
References:
Toray Industries, Inc.;Osumi, Kazuya;Matsumura, Yuki;Hayashi, Shinnosuke;Hoshi, Masaki;Vallet, Martial;Yokosaka, Shinya;Aoki, Takumi;Meguro, Hiroyuki;Kaino, Mie;Takagaki, Kozuc;Sasaki, Rie US2020/392107, 2020, A1 Location in patent:Paragraph 0423-0425