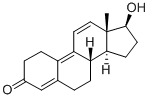
Trenbolone synthesis
- Product Name:Trenbolone
- CAS Number:10161-33-8
- Molecular formula:C18H22O2
- Molecular Weight:270.37
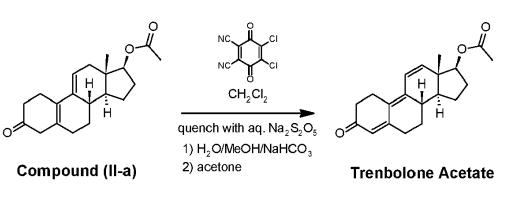
Synthesis of Trenbolone acetate
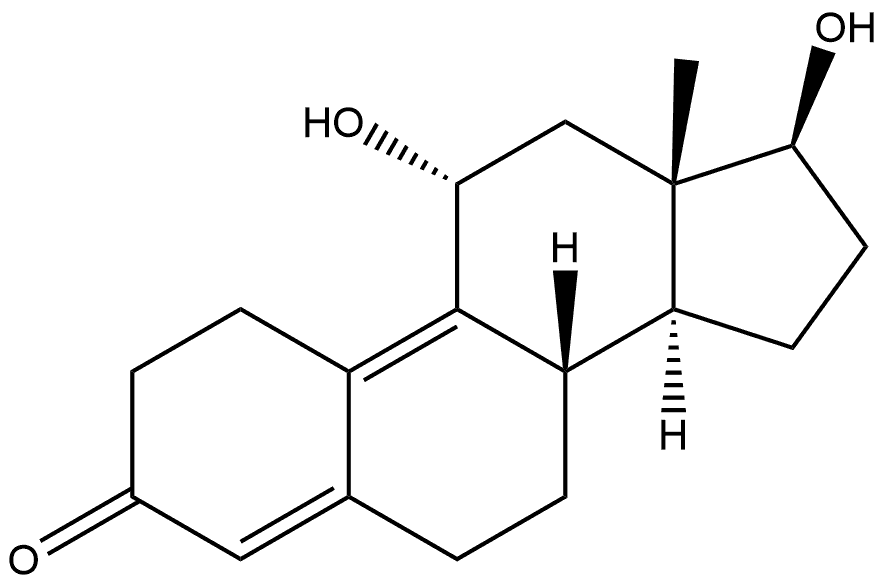
117605-69-3
0 suppliers
inquiry

10161-33-8
222 suppliers
$43.00/1mg
Yield:10161-33-8 90%
Reaction Conditions:
with toluene-4-sulfonic acid in chloroform at 20; for 2 h;
References:
Peng, Yaqin;Gao, Chenghua;Zhang, Zili;Wu, Shijie;Zhao, Jing;Li, Aitao [ACS Catalysis,2022,vol. 12,# 5,p. 2907 - 2914]
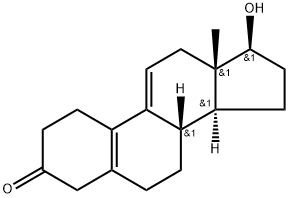
5218-51-9
7 suppliers
inquiry

10161-33-8
222 suppliers
$43.00/1mg
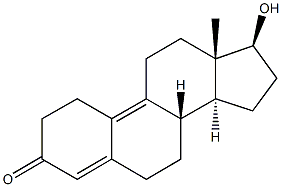
6218-29-7
126 suppliers
$16.00/200mg

10161-33-8
222 suppliers
$43.00/1mg
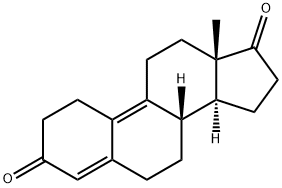
5173-46-6
247 suppliers
$69.00/5g

10161-33-8
222 suppliers
$43.00/1mg
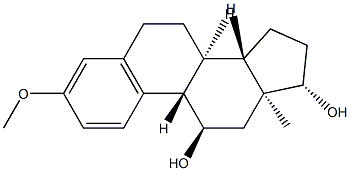
10516-35-5
0 suppliers
inquiry

10161-33-8
222 suppliers
$43.00/1mg