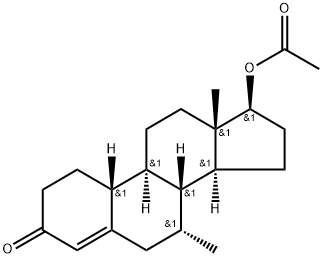
trestolone acetate synthesis
- Product Name:trestolone acetate
- CAS Number:6157-87-5
- Molecular formula:C21H30O3
- Molecular Weight:330.47
2590-41-2
176 suppliers
$50.00/1g
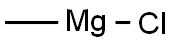
676-58-4
291 suppliers
$15.00/25ml

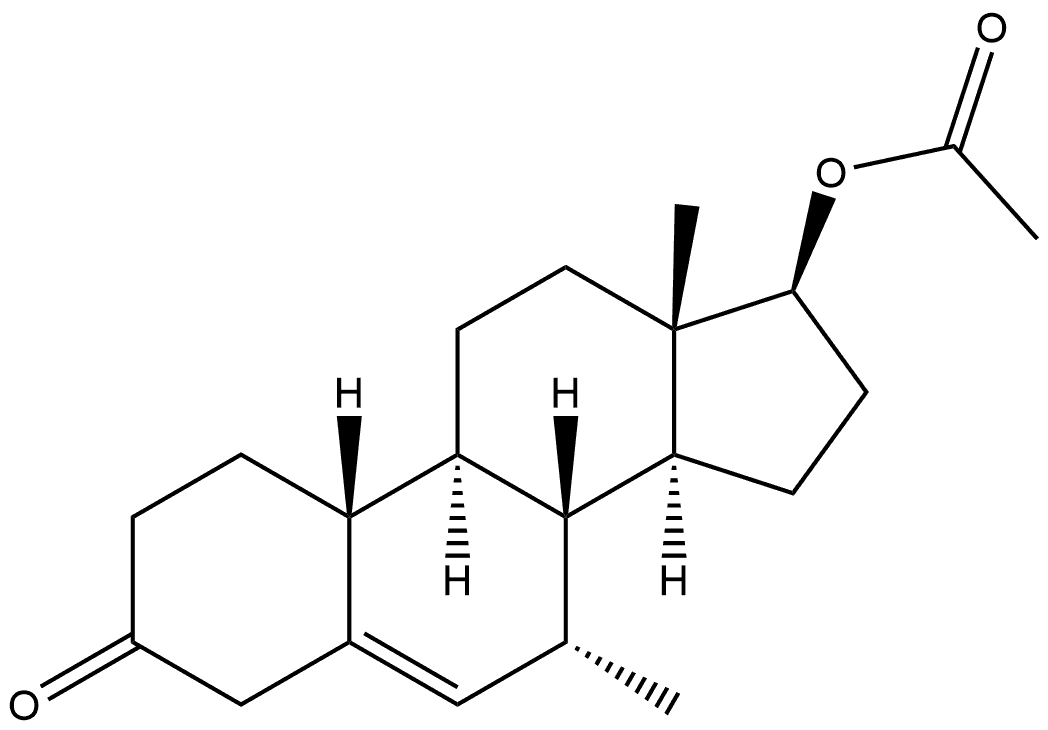
6157-87-5
112 suppliers
inquiry
Yield:6157-87-5 78%
Reaction Conditions:
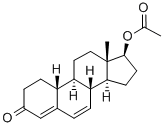
Stage #1: 6-dehydro-19-nortestosterone acetate;methylmagnesium chloride;copper diacetate in tetrahydrofuran at -45 - -35; for 3 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 10; for 0.5 h;
Steps:
3 Example 3
Tetrahydrofuran (717.6 g), compound (5A) (204 g) and anhydrous copper (II) acetate (23. 6 G) were charged to A suitable vessel. The slurry was stirred and cooled to BETWEEN-45 C AND-35 G. Methyl-magnesium chloride solution (23% IN THF, ASSAYED 22. 6%, 346. 1 g) was then added slowly at such a rate to maintain the reaction temperature BETWEEN-45 C AND-35 C OVER A minimum of three hours. After completion of the addition, the reaction mixture was stirred AT-45 C to-35°C and monitored by HPLC. The mixture was then quenched with 37% HYDROCHLORIC ACID (128. 1 G) LEEPING THE TEMPERATURE below 10°C. The mixture was maintained below 10°C for 30 minutes. Water (408 g) was slowly added over A period of about 20 minutes. Heptane (428. 2 g) was added and the mixture allowed to warm to ambient temperature. The aqueous layer was separated and the product was extracted with heptane. The combined organic extracts were washed with 25% ammonium hydroxide solution and purified water. The solvent was distilled under atmospheric pressure until approximately 3 volumes [with respect to the input weight of compound (5A) ] remained. Tert-butyl methyl ether was added and the mixture cooled to crystallise the product. The product was isolated by filtration and dried at 40-50 C (yield: 78%; a: 6 ratio = 99).
References:
WO2004/78774,2004,A1 Location in patent:Page 34
54793-00-9
0 suppliers
inquiry

6157-87-5
112 suppliers
inquiry
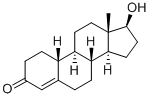
434-22-0
253 suppliers
$33.00/1mg

6157-87-5
112 suppliers
inquiry
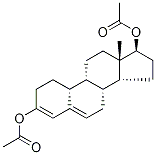
4999-76-2
9 suppliers
inquiry

6157-87-5
112 suppliers
inquiry

2590-41-2
176 suppliers
$50.00/1g

6157-87-5
112 suppliers
inquiry