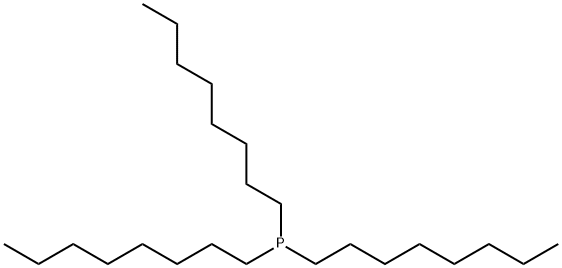
Trioctylphosphine synthesis
- Product Name:Trioctylphosphine
- CAS Number:4731-53-7
- Molecular formula:C24H51P
- Molecular Weight:370.64
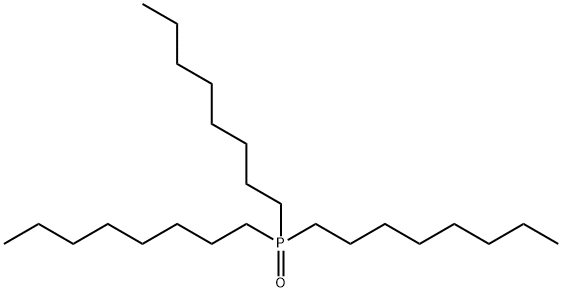
78-50-2
252 suppliers
$18.00/5g

4731-53-7
220 suppliers
$11.19/5ML
Yield:-
Reaction Conditions:
with indium(III) bromide;1,1,3,3-Tetramethyldisiloxane in toluene at 100; for 18 h;Inert atmosphere;Sealed tube;
Steps:
4.2. General procedure for the preparation of compounds 2b to 2f and 2i to 2l
General procedure: In a sealed tube containing a solution of the phosphine oxide derivative (5 mmol) in anhydrous toluene (1 M) was added InBr3 (1 mol %, 0.05 mmol) and the TMDS (1.5 equiv, 7.5 mmol) under an argon atmosphere. The reaction mixture was stirred at 100 °C during 7-40 h depending on the substrate (the reaction was monitored by 31P NMR). After complete consumption of the reagent the mixture was kept under argon in the sealed tube and cooled to 0 °C. A solution of BH3.SMe2 (2 M in THF, 1 equiv) was then slowly added. After 1 h at room temperature, 31P NMR analysis of an aliquot indicates complete conversion to phosphine borane adduct. The crude was poured in an erlenmeyer flask and silica gel was carefully added while stirring. When silica gel was added in the reaction mixture, a slightly exothermic reaction was observed. The reaction mixture was then filtered on silica gel and washed several times with ethyl acetate. After evaporation of the ethyl acetate, the residue was purified by flash chromatography on silica gel with pure cyclohexane to afford the desired phosphine-borane.
References:
Pehlivan, Leyla;Métay, Estelle;Delbrayelle, Dominique;Mignani, Gérard;Lemaire, Marc [Tetrahedron,2012,vol. 68,# 15,p. 3151 - 3155] Location in patent:supporting information; experimental part
24219-37-2
4 suppliers
inquiry

4731-53-7
220 suppliers
$11.19/5ML

111-66-0
173 suppliers
$10.00/10 mL

4731-53-7
220 suppliers
$11.19/5ML

111-83-1
504 suppliers
$12.00/25g

4731-53-7
220 suppliers
$11.19/5ML