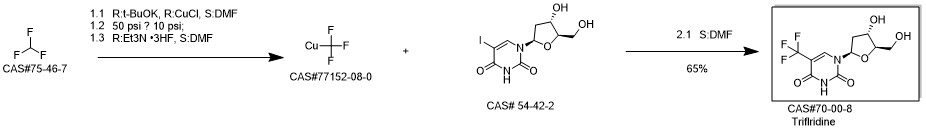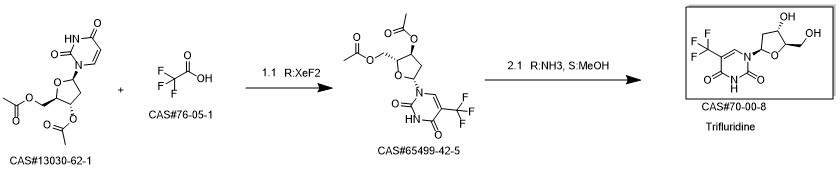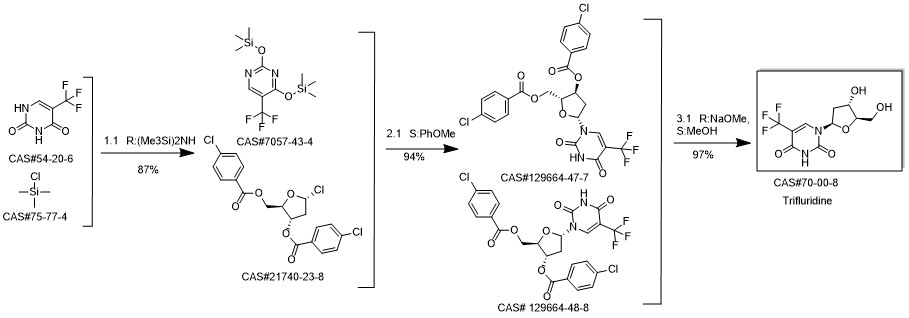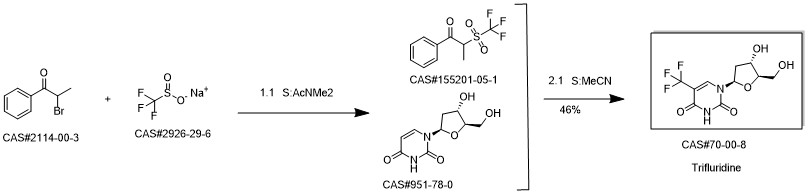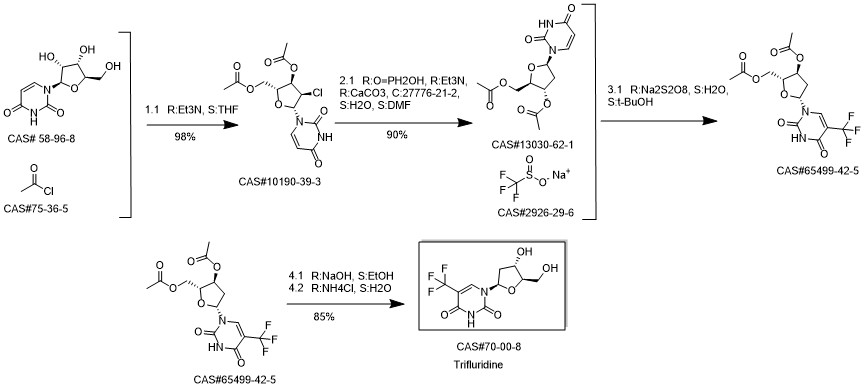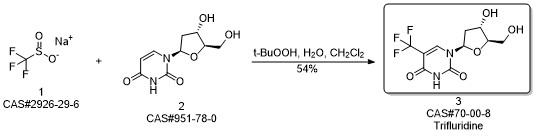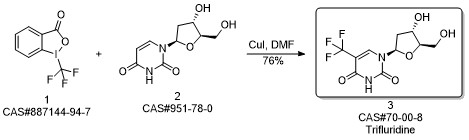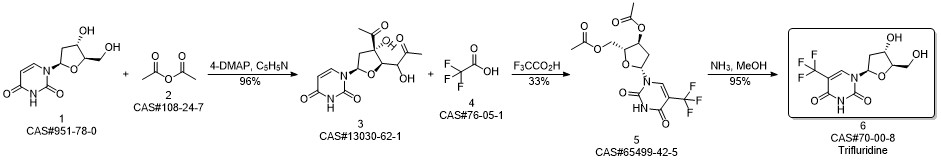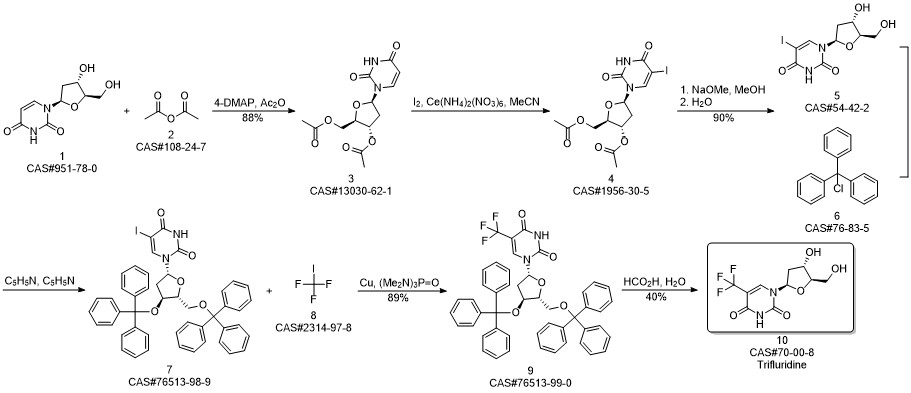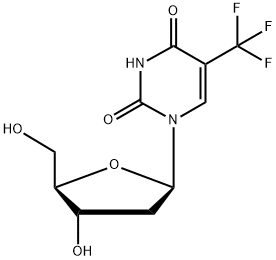
Trifluridine synthesis
- Product Name:Trifluridine
- CAS Number:70-00-8
- Molecular formula:C10H11F3N2O5
- Molecular Weight:296.2
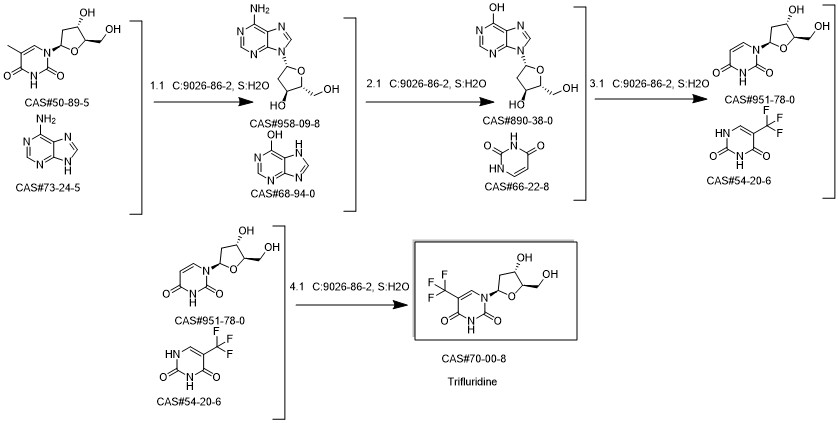
Reference: Fernandez-Lucas, Jesus; Fresco-Taboada, Alba; Acebal, Carmen; Mata, Isabel; Arroyo, Miguel. Applied Microbiology and Biotechnology. Enzymatic synthesis of nucleoside analogues using immobilized 2'-deoxyribosyltransferase from Lactobacillus reuteriVolume. 91. Issue 2. Pages 317-327. Journal; Online Computer File (2011)
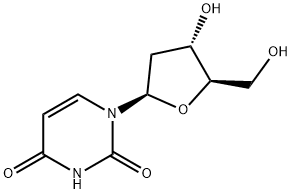
951-78-0
512 suppliers
$5.00/100mg
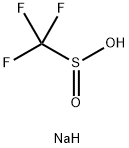
2926-29-6
310 suppliers
$6.00/5g

70-00-8
391 suppliers
$32.00/50mg
Yield:70-00-8 94.3%
Reaction Conditions:
with tert.-butylhydroperoxide in water at -3 - 60; for 2 h;Inert atmosphere;Temperature;
Steps:
2
456 g (2 mol) of 2'-deoxyuridine and 985.2 g (6 mol)95% sodium trifluoromethanesulfinate was added to 7.98 L of purified water, stirred down to -3 ° C, protected by nitrogen stream,After stirring, 772 g (6 mol) of 70% t-butyl hydroperoxide was added dropwise, and the temperature was less than 5 ° C during the dropwise addition,After the dropwise addition, the temperature was raised to 60 ° C, the reaction was stirred for 2 hours, and the reaction was completed.Drop to room temperature, respectively, 8L ethyl acetate extraction 3 times, the combined extract,50 deg.] C and concentrated under reduced pressure to give 558g trifluridine, yield: 94.3%, purity: 97.2%.
References:
CN104761602,2017,B Location in patent:Paragraph 0034; 0035
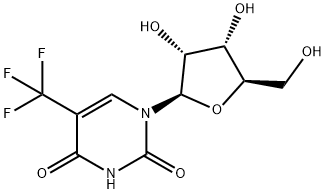
21618-67-7
29 suppliers
inquiry

70-00-8
391 suppliers
$32.00/50mg
![1-[2-deoxy-3,5-di-O-(p-chlorobenzoyl)-β-D-erythropentofuranosyl]-5-trifluoromethyl uracil](/CAS/20210111/GIF/129664-47-7.gif)
129664-47-7
4 suppliers
inquiry

70-00-8
391 suppliers
$32.00/50mg
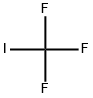
2314-97-8
183 suppliers
$40.00/25g

951-78-0
512 suppliers
$5.00/100mg

70-00-8
391 suppliers
$32.00/50mg

951-78-0
512 suppliers
$5.00/100mg
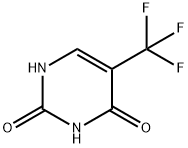
54-20-6
332 suppliers
$6.00/250mg

70-00-8
391 suppliers
$32.00/50mg