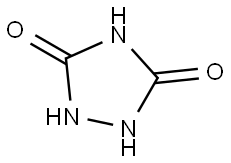
URAZOLE synthesis
- Product Name:URAZOLE
- CAS Number:3232-84-6
- Molecular formula:C2H3N3O2
- Molecular Weight:101.06
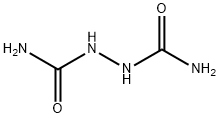
872-50-4
986 suppliers
$10.00/100ML
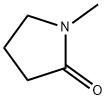
110-21-4
124 suppliers
$8.00/1g

3232-84-6
67 suppliers
$45.00/50mg
Yield: 89%
Reaction Conditions:
in water;acetone
Steps:
5 EXAMPLE 5
EXAMPLE 5 400 g of N-methyl-pyrrolidone and 160 g of hydrazodicarbonamide still moist from the suction filter (contains 73.8% of dry hydrazodicarbonamide) are heated to 205° C. in a one-liter stirrer apparatus similar to that of Example 1. The ammonia which begins to be released at 160° C. is expelled with nitrogen. After a reaction time of 3.5 hours at 205° C., about 300 g of solvent are distilled off in a water jet vacuum and acetone is added to the solution remaining behind. Triazolidine-3,5-dione which crystallises out is suction-filtered after it has cooled to room temperature and is then washed with acetone and dried. 74 g of a 97.5% pure 1,2,4-triazolidine-3,5-dione are obtained. A further 16 g of a 96.5% 1,2,4-triazolidine-3,5-dione can be isolated from the mother liquor, so that the total yield is 90 g (89% of the theoretical yield).
References:
Bayer Aktiengesellschaft US4323687, 1982, A

110-21-4
124 suppliers
$8.00/1g

3232-84-6
67 suppliers
$45.00/50mg
19617-43-7
57 suppliers
$95.00/250mg

3232-84-6
67 suppliers
$45.00/50mg

110-21-4
124 suppliers
$8.00/1g
90-12-0
288 suppliers
$17.00/25mL

3232-84-6
67 suppliers
$45.00/50mg