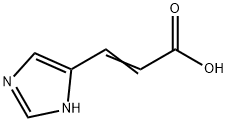
Urocanic acid synthesis
- Product Name:Urocanic acid
- CAS Number:104-98-3
- Molecular formula:C6H6N2O2
- Molecular Weight:138.12
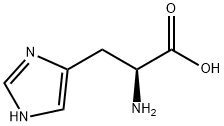
71-00-1
814 suppliers
$7.00/5g

104-98-3
183 suppliers
$44.50/5g
Yield:-
Reaction Conditions:
with histidase EC 4.3.1.3;Tris buffer in water; pH=9.0 at 37; for 24 h;
References:
Sacks, Gavin L.;Brenna, J. Thomas [Analytical Chemistry,2005,vol. 77,# 4,p. 1013 - 1019]