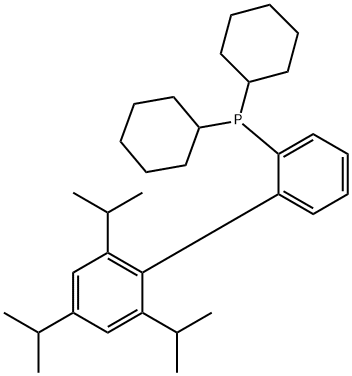
X-PHOS synthesis
- Product Name:X-PHOS
- CAS Number:564483-18-7
- Molecular formula:C33H49P
- Molecular Weight:476.72
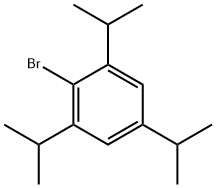
21524-34-5
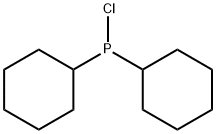
16523-54-9
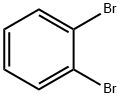
583-53-9

564483-18-7
Under nitrogen protection, 200 mL of tetrahydrofuran (THF) and 31 g of 2-bromo-1,3,5-triisopropylbenzene were added to a 1 L three-necked flask. The reaction mixture was cooled to 0 °C and 46 mL of a 2.5 M hexane solution of n-butyllithium was slowly added dropwise. After the dropwise addition was completed, the reaction continued to be stirred at 0 °C for 1 hour. Subsequently, 24.6 g of 1,2-dibromobenzene was slowly added dropwise at the same temperature. After completion of the dropwise addition, the reaction was continued at 0°C for 1 hour. Next, 42 mL of 2.5 M hexane solution of n-butyllithium was added dropwise to the reaction mixture at 0 °C. After the dropwise addition was completed, the reaction was continued with stirring at 0 °C for 1 hour. Then, 26.7 g of dicyclohexylphosphonium chloride was slowly added dropwise and the reaction mixture was allowed to warm up to room temperature naturally and the reaction was continued to be stirred for 2 hours. Upon completion of the reaction, 200 mL of saturated aqueous ammonium chloride solution was slowly added dropwise to quench the reaction under the cooling of an ice bath. After performing phase separation, the organic phase was concentrated and crystallized by adding methanol. The solid was collected by filtration to afford 44.5 g of a white crystalline product of 2-dicyclohexylphosphino-2',4',6'-triisopropylbiphenyl in 89% yield.

21524-34-5
278 suppliers
$6.00/5g

16523-54-9
273 suppliers
$8.00/1g

583-53-9
321 suppliers
$10.00/5g

564483-18-7
400 suppliers
$5.00/250mg
Yield:564483-18-7 89%
Reaction Conditions:
Stage #1: 2,4,6-triisopropyl-1-bromobenzenewith n-butyllithium in tetrahydrofuran at 0; for 1 h;Inert atmosphere;
Stage #2: 2,3-dibromobenzene in tetrahydrofuran at 0; for 1 h;Inert atmosphere;
Stage #3: chlorodicyclohexylphosphaneFurther stages;
Steps:
4 Example 4
Under the protection of nitrogen, 200 ml of THF and 31g of 2, 4, 6-tri-isopropyl-bromobenzene was added to 1L three-mouth bottle. The temperature was cooled down to 0 °C, and 46 ml of 2.5M n-butyl lithiumwas added dropwise. The reaction was continued for 1 hour after the addition. At 0 °C, 24.6g O-dibromobenzene was added dropwise. The reaction was continued for 1 hour after the addition. At0 °C, 42 ml of 2.5M n-butyl lithiumwas added dropwise to the reaction liquid.After the addition, the reaction was continued for 1 hour. 26.7g of dicyclohexyl phosphine was added dropwise, and the temperature was naturally warmed to react at room temperature for 2 hours. 200 ml saturated ammonium chloride aqueous solution was added dropwsie to the reaction liquid under the ice bath to quench the reaction. After phase separation, the organic phase was precipitated, and methanol was added for crystallization. Byfiltering, 44.5g white product of 2’-dicyclohexyl phosphino-2, 4, 6-tri-isopropyl biphenyl was obtained, with a yield of 89%.
References:
CN105273006,2016,A Location in patent:Paragraph 0069; 0070
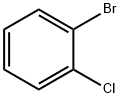
694-80-4
403 suppliers
$5.00/10g

21524-34-5
278 suppliers
$6.00/5g

16523-54-9
273 suppliers
$8.00/1g

564483-18-7
400 suppliers
$5.00/250mg

694-80-4
403 suppliers
$5.00/10g

21524-34-5
278 suppliers
$6.00/5g
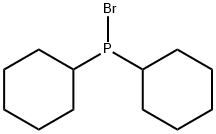
100384-03-0
2 suppliers
inquiry

564483-18-7
400 suppliers
$5.00/250mg