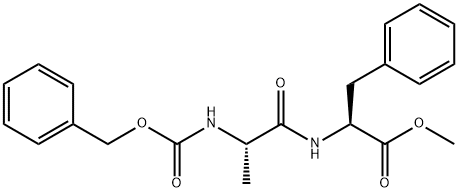
Z-ALA-PHE-OME synthesis
- Product Name:Z-ALA-PHE-OME
- CAS Number:3235-14-1
- Molecular formula:C21H24N2O5
- Molecular Weight:384.43
Yield:3235-14-1 97%
Reaction Conditions:
with 1-propanephosphonic acid cyclic anhydride;N-ethyl-N,N-diisopropylamine in 1,4-dioxane at 20; for 4 h;Inert atmosphere;
Steps:
General Procedure for the Formation of Carboxylatesand Subsequent Coupling Reaction
General procedure: To a solution of Cbz-Ala-Phe-OMe (0.3 g, 0.81 mmol) in dioxane (2 mL) at r.t.were added LiOH (25 mg, 1 mmol) and H2O (0.35 mL, 19.44mmol, 24 molar equiv). Stirring at r.t. and monitoring byHPLC showed complete saponification and a clear,homogeneous solution generally after 3 h.Amino acid H-AA-OMe, HCl (0.9 mmol) and HATU (0.463g, 1.22 mmol) were added and the mixture was stirred at r.t.until completion, generally 4 h.The diastereomeric purity was measured at this stage, beforeisolation and purification.After concentration in vacuo, the residue was purified bysilica gel chromatography with a mixture of CH2Cl2-EtOAcas eluent to afford the pure tripeptide as a white solid.
References:
Azzouz, Rabah;Petit, Sylvain;Rouchet, Jean-Baptiste;Bischoff, Laurent [Synlett,2014,vol. 25,# 13,art. no. 3909,p. 1843 - 1846] Location in patent:supporting information
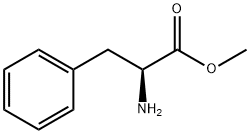
1142-20-7
467 suppliers
$5.00/1g
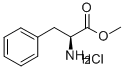
2577-90-4
92 suppliers
$102.00/1g
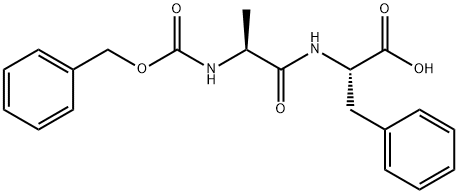
2768-53-8
61 suppliers
$40.00/1G

3235-14-1
17 suppliers
inquiry

1142-20-7
467 suppliers
$5.00/1g

3235-14-1
17 suppliers
inquiry